Preparation method for cefcapene pivoxil hydrochloride
A technology of cefcapine hydrochloride and hydrochloric acid, applied in the field of medicine and chemical industry, can solve the problems of unsuitability for large-scale production, low yield, complicated operation and the like, and achieves the advantages of simple operation, improved reaction yield and improved reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
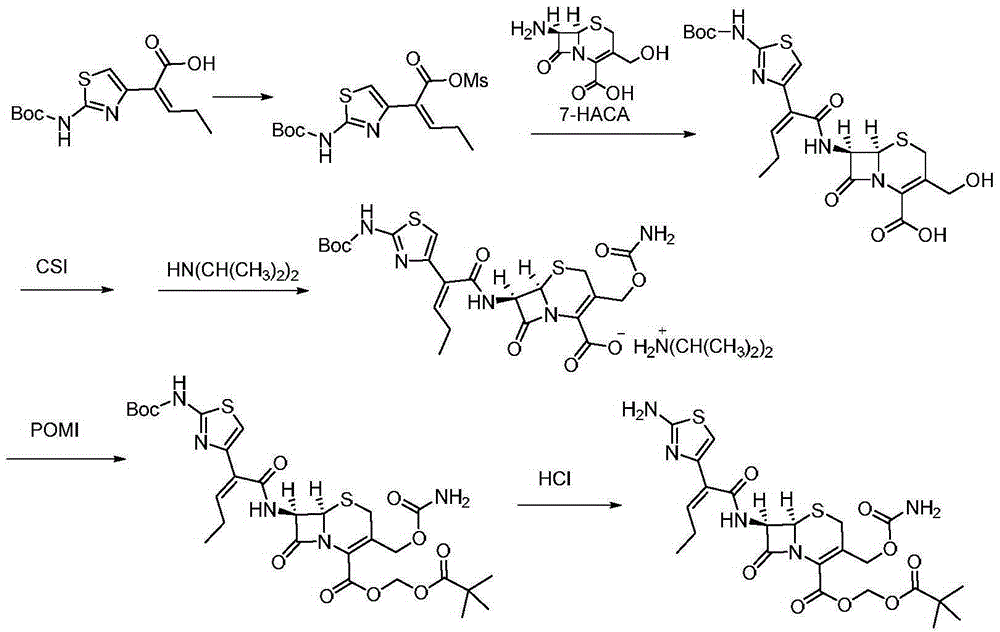
[0032] A preparation method of cefcapene pivoxil hydrochloride, the method may further comprise the steps:
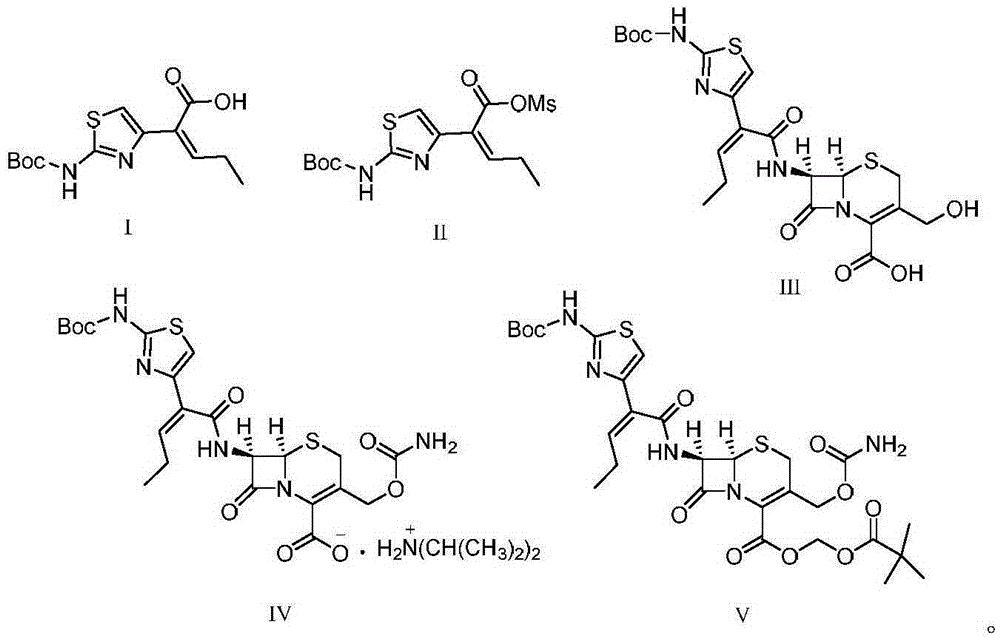
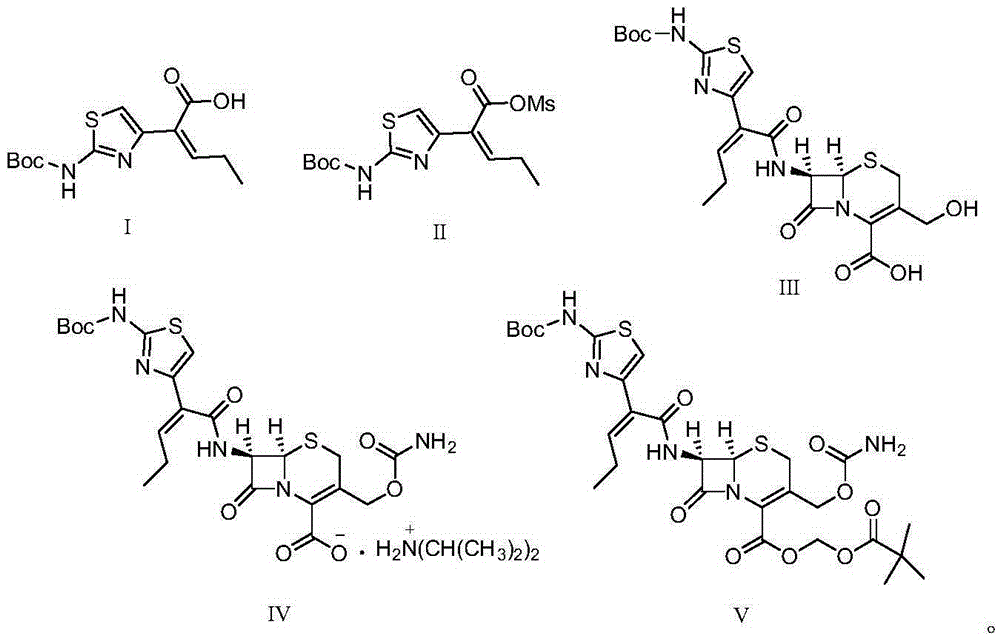
[0033] 1) 29.8 g (100 mmol) of the compound represented by formula I ((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid)) was stirred and dissolved in 80 mL of pyridine, Add 17.2 g (150 mmol) of methanesulfonyl chloride at -15°C, stir and react for 1 hour, then filter to obtain a liquid containing the compound represented by formula II, store at -10°C, and set aside;
[0034]2) First mix 6.2g of proline, 10.1g (100mmol) of diisopropylamine and 20.7g (90mmol) of 7-HACA in 100mL of methanol and lower the temperature to 0°C, then mix the product obtained in step 1) containing the formula II The liquid of the compound was dripped into the reaction for 1.5h, naturally rose to room temperature, adjusted the pH of the solution to 5 with 2mol / L hydrochloric acid, extracted with dichloromethane, combined the organic phases, concentrated under reduced pressure, recr...
Embodiment 2
[0039] A preparation method of cefcapene pivoxil hydrochloride, the method may further comprise the steps:
[0040] 1) 29.8 g (100 mmol) of the compound represented by formula I ((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid)) was stirred and dissolved in 100 mL of pyridine, Add 14.9 g (130 mmol) of methanesulfonyl chloride at 0°C, stir and react for 2 hours, then filter to obtain a liquid containing the compound represented by formula II, put it at 0°C, and set aside;
[0041] 2) First mix 4.6g of proline, 11.1g (110mmol) of diisopropylamine (110mmol) and 18.4g (80mmol) of 7-HACA in methanol and lower the temperature to 5°C, then the The liquid of the compound was dropped into the reaction for 2 hours, and naturally rose to room temperature, and the pH of the solution was adjusted to 5 with 2mol / L hydrochloric acid, extracted with dichloromethane, the organic phases were combined, concentrated under reduced pressure, recrystallized from methanol, and dried t...
Embodiment 3
[0046] A preparation method of cefcapene pivoxil hydrochloride, the method may further comprise the steps:
[0047] 1) 29.8 g (100 mmol) of the compound represented by formula I ((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid)) was stirred and dissolved in 120 mL of pyridine, Add 16 g (140 mmol) of methanesulfonyl chloride at -10°C, stir and react for 1 hour, then filter to obtain a liquid containing the compound represented by formula II, store it at -15°C, and set aside;
[0048] 2) First mix 5.5g of proline, 12.1g (120mmol) of diisopropylamine (120mmol) and 19.6g (85mmol) of 7-HACA in methanol and lower the temperature to 10°C, then the The liquid of the compound was dropped into the reaction for 2 hours, and naturally rose to room temperature, and the pH of the solution was adjusted to 5 with 2mol / L hydrochloric acid, extracted with dichloromethane, the organic phases were combined, concentrated under reduced pressure, recrystallized from methanol, and dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com