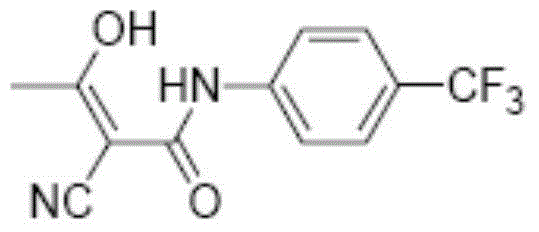
Method for synthesizing teriflunomide
A synthesis method and technology of teriflunomide are applied in the field of pharmaceutical synthesis, and can solve the problems of being unsuitable for large-scale industrial production, inflammable and explosive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of teriflunomide with cyanoacetic acid as initial raw material
[0052] Add 3.0 g of cyanoacetic acid to 130 mL of thionyl chloride, reflux at 75°C for 2 hours, remove excess thionyl chloride on a rotary evaporator to obtain a yellow oil, add anhydrous dichloromethane to dissolve, and obtain a yellow oil Anhydrous dichloromethane solution, set aside.
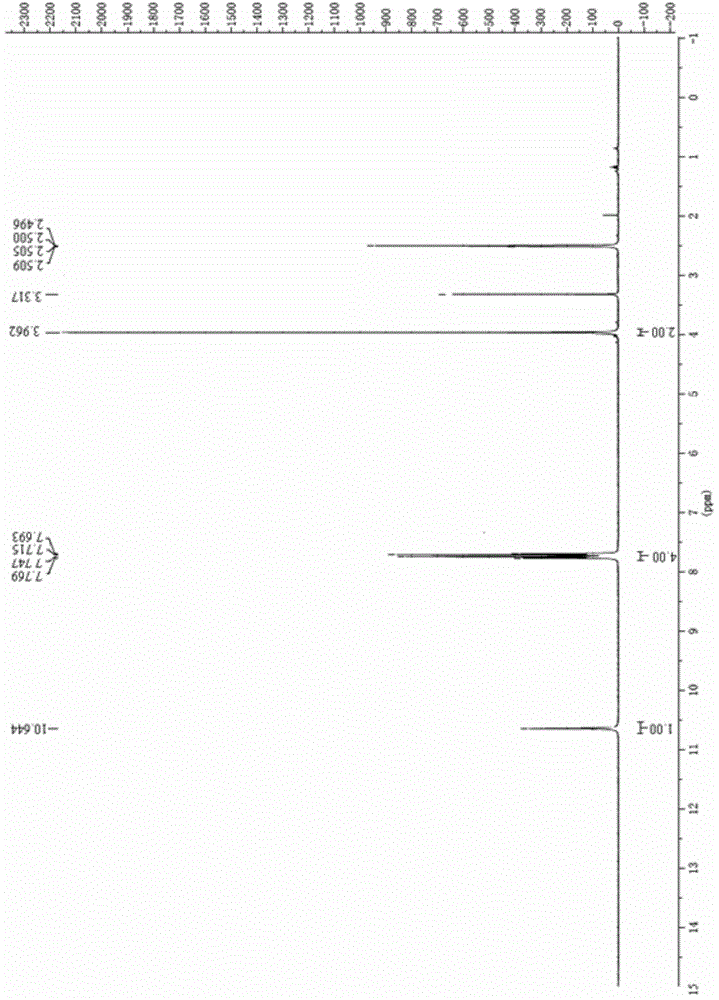
[0053] Dissolve 4.40 mL of p-trifluoromethylaniline in anhydrous dichloromethane, add 7.32 mL of triethylamine, and add the obtained yellow oil in anhydrous dichloromethane dropwise at 0°C. Stir overnight at °C, remove the solvent, and recrystallize the crude product from ethanol to obtain 4.51 g of solid, with a yield of 57%. Gained solid is carried out proton nuclear magnetic resonance spectrum detection, gained data is 1 H-NMR(400MHz,DMSO)δ10.64(s,1H),7.76(d,J=8.7Hz,2H),7.70(d,J=8.9Hz,2H),3.96(s,2H), such as figure 1 As shown, the measured data is consistent with the 2-cyano-N-(4-trifluoromethy...
Embodiment 2
[0055] Example 2 The preparation of teriflunomide with cyanoacetic acid as the initial raw material
[0056] Take 3.0 g of cyanoacetic acid and add it to 130 mL of thionyl chloride, reflux at 75 ° C for 1 h, remove excess thionyl chloride on a rotary evaporator to obtain a yellow oil, add chloroform to dissolve, and obtain a chloroform solution of the yellow oil. spare.
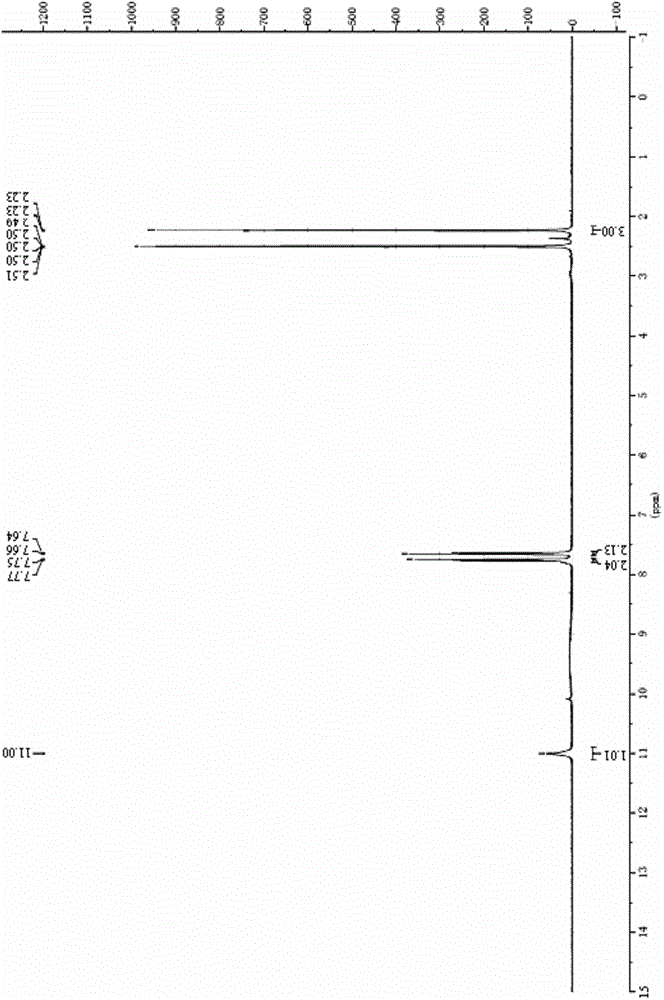
[0057] Dissolve 4.40 mL of p-trifluoromethylaniline in chloroform, add 7.32 mL of triethylamine, and add dropwise at 0°C to obtain a solution of yellow oil in chloroform. After the dropwise addition, stir at 0-50°C for 1 h, The solvent was removed, and the crude product was recrystallized from ethanol to obtain 4.82 g of solid, with a yield of 61%. Gained solid is carried out proton nuclear magnetic resonance spectrum detection, gained data is 1 H-NMR (400MHz, DMSO) δ10.64(s, 1H), 7.76(d, J=8.7Hz, 2H), 7.70(d, J=8.9Hz, 2H), 3.96(s, 2H), measured The data are consistent with the 2-cyano-N-(4-trifluoromethyl...
Embodiment 3
[0059] Example 3 Preparation of teriflunomide with cyanoacetic acid as initial raw material
[0060] Take 3.0 g of cyanoacetic acid and add it to 130 mL of thionyl chloride, reflux at 85 ° C for 8 h, remove excess thionyl chloride on a rotary evaporator to obtain a yellow oil, add carbon tetrachloride to dissolve, and obtain a yellow oil Carbon tetrachloride solution, spare.
[0061] Dissolve 4.40 mL of p-trifluoromethylaniline in carbon tetrachloride, add 7.32 mL of triethylamine, and add dropwise at 0°C to obtain a solution of yellow oil in carbon tetrachloride. After stirring for 2 h, the solvent was removed, and the crude product was recrystallized from ethanol to obtain 4.22 g of solid, with a yield of 53%. Gained solid is carried out proton nuclear magnetic resonance spectrum detection, gained data is 1 H-NMR (400MHz, DMSO) δ10.64(s, 1H), 7.76(d, J=8.7Hz, 2H), 7.70(d, J=8.9Hz, 2H), 3.96(s, 2H), measured The data are consistent with the 2-cyano-N-(4-trifluoromethylphen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com