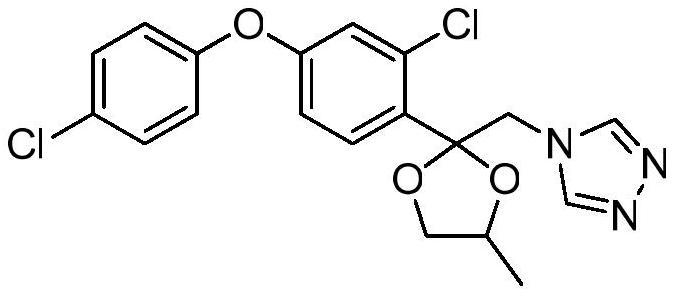
A kind of preparation method of difenoconazole nitrate
A technology of difenoconazole and nitrate, applied in directions such as organic chemistry, can solve the problems of many solid wastes, severe reaction conditions, and high reaction temperature, and achieve the effects of improving safety, short reaction time, and increasing the content of crude products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
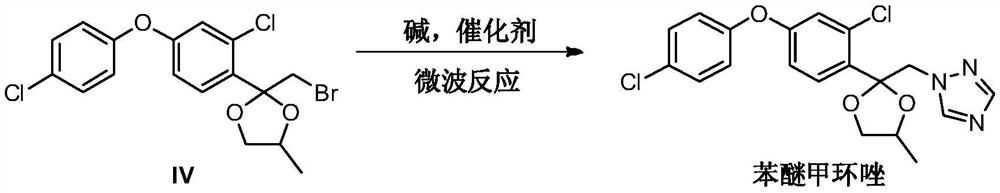
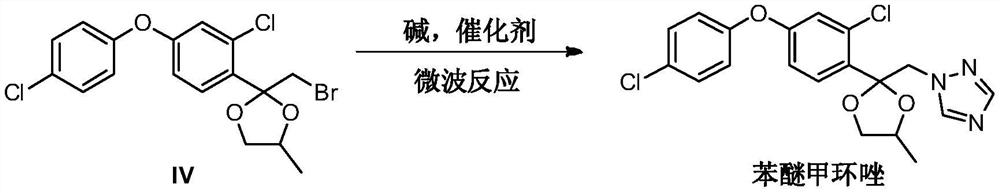
[0034] Add 2-(bromomethyl)-2-(2-chloro-4-(4-chlorophenoxy)phenyl)-4-methyl-1,3-dioxolane ( 4g, 9.57mmol) and 1H-1,2,4 triazole (0.66g, 9.57mmol), potassium carbonate (1.32g, 9.57mmol), potassium iodide (0.16g, 0.96mmol) and toluene 20mL, placed in microwave reaction The reaction was carried out at 160° C. for 15 minutes in an apparatus, and the reaction yield was 85.2% by liquid phase monitoring.
Embodiment 2
[0036] Add 2-(bromomethyl)-2-(2-chloro-4-(4-chlorophenoxy)phenyl)-4-methyl-1,3-dioxolane ( 4g, 9.57mmol) and 1H-1,2,4 triazole (0.8g, 11.48mmol), potassium carbonate (2.64g, 14.35mmol), potassium iodide (0.40g, 2.39mmol) and N,N-dimethyl Formamide 20mL was placed in a microwave reactor and reacted at 185°C for 15min, and the reaction yield was 92.6% by liquid phase monitoring.
Embodiment 3
[0038] Add 2-(bromomethyl)-2-(2-chloro-4-(4-chlorophenoxy)phenyl)-4-methyl-1,3-dioxolane ( 4g, 9.57mmol) and 1H-1,2,4 triazole (1.0g, 14.35mmol), potassium carbonate (1.98g, 19.14mmol), potassium iodide (0.80g, 4.78mmol) and N-methylpyrrolidone 20mL, Placed in a microwave reactor and reacted at 190°C for 15 minutes, the reaction yield was 91.8% by liquid phase monitoring.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com