A method for preparing n-substituted-1,2,3,6-tetrahydropyridine
A technology of tetrahydropyridine and piperidinol, applied in organic chemistry and other directions, can solve problems such as restricting amplification synthesis, unfriendly environment, irritating odor, etc., and achieve the effect of improving market competitiveness, environmental friendliness, and avoiding high temperature reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
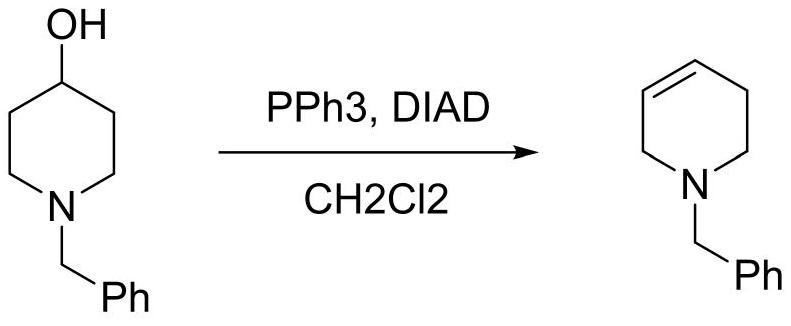
[0016] Synthesis of N-Benzyl-1,2,3,6-tetrahydropyridine
[0017]
[0018] In a 500mL three-necked flask, dissolve N-benzyl-4-piperidinol (38.3g, 0.2mol) and triphenylphosphine (78.7g, 0.3mol) in 400mL of dichloromethane, and Add diisopropyl azodicarboxylate (60.7 g, 0.3 mol) dropwise, and stir at room temperature for 4 hours. After the reaction, cool down to below -20°C, stir to precipitate a solid, filter, and filter out PPh3O / (NHCO2i- Pr)2 complex 89.7g, the filtrate distilled off the solvent, added n-heptane, stirred at 0°C for 1 hour, filtered out 4.8g of the solid complex, concentrated the filtrate to remove the solvent, and distilled under reduced pressure to obtain a colorless oily substance N- Benzyl-1,2,3,6-tetrahydropyridine 28.8g (95-98℃ / 5mmHg), yield 83.5%, GC: 98.7%, 1 HNMR (400MHz, CDCl3): δ2.15-2.22 (2H, m), 2.58 (t, J = 5.6, 2H), 2.97-3.01 (2H, m), 3.60 (2H, s), 5.65-5.71 (1H ,m), 5.74-5.81(1H,m), 7.24-7.40(5H,m).
Embodiment 2
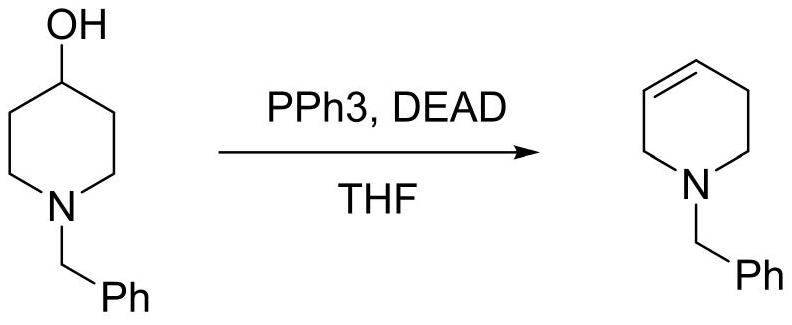
[0020] Synthesis of N-Benzyl-1,2,3,6-tetrahydropyridine
[0021]
[0022] In a 500mL three-necked flask, dissolve N-benzyl-4-piperidinol (38.3g, 0.2mol) and triphenylphosphine (63.0g, 0.24mol) in 400mL tetrahydrofuran, and add dropwise Diethyl azodicarboxylate (41.8g, 0.24mol), after dropping, stir at room temperature for 4 hours, after the reaction, cool down to below -20°C, stir to precipitate a solid, filter, filter out PPh3O / (NHCO2Et)2 complex 80.5 g of compound, the filtrate distilled off the solvent, added n-hexane, stirred at 0°C for 1 hour, filtered out 6.1 g of the solid complex, concentrated the filtrate to remove the solvent, and distilled under reduced pressure to obtain a colorless oily substance N-benzyl-1,2 , 3,6-tetrahydropyridine 28.5g (95-98°C / 5mmHg), yield 82.3%, GC: 98.8%.
Embodiment 3
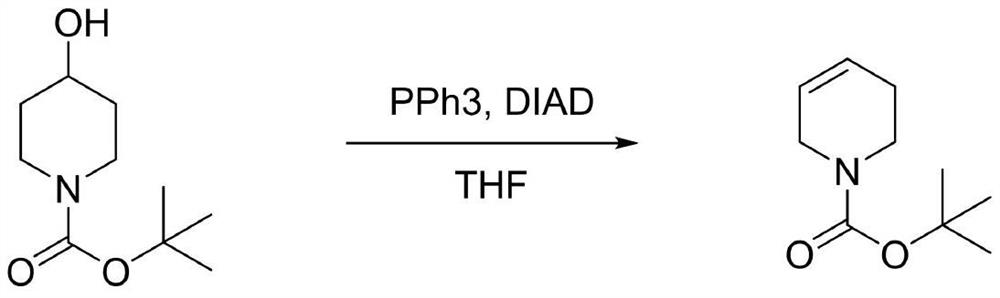
[0024] Synthesis of N-Boc-1,2,3,6-tetrahydropyridine
[0025]
[0026] In a 500mL three-necked flask, dissolve N-Boc-4-piperidinol (40.3g, 0.2mol) and triphenylphosphine (104.9g, 0.4mol) in 400mL tetrahydrofuran, and add Diisopropyl nitrogen dicarboxylate (80.9g, 0.4mol), after dropping, stir at room temperature for 4 hours, after the reaction, cool down to below -20°C, stir to precipitate a solid, filter, and filter out PPh3O / (NHCO2i-Pr)2 Complex 80.0g, the filtrate distilled off the solvent, added n-heptane, stirred at 0°C for 1 hour, filtered out 6.9g of the solid complex, concentrated the filtrate to remove the solvent, and distilled under reduced pressure to obtain a light yellow oil N-Boc-1 ,2,3,6-Tetrahydropyridine 31.2g (55-57℃ / 3mmHg), yield 85.1%, GC detection: purity 98.5%, 1 H-NMR (400MHz, CDCl3): δ5.83–5.74(m,1H),5.67–5.57(m,1H),3.84(2H),3.45(2H),2.09(m,2H),1.44(s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com