Nickel titanate doped lithium aluminum hydride hydrogen-storage material and preparation method thereof
A technology of lithium aluminum hydride and hydrogen storage materials, applied in chemical instruments and methods, hydrogen, inorganic chemistry, etc., can solve the problems of MgH2 catalytic effect difference, reduced hydrogen release amount, high hydrogen release temperature, etc., and achieve low cost and high hydrogen release The effect of performance improvement and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A preparation method of nickel titanate doped lithium aluminum hydride hydrogen storage material, comprising the following steps:
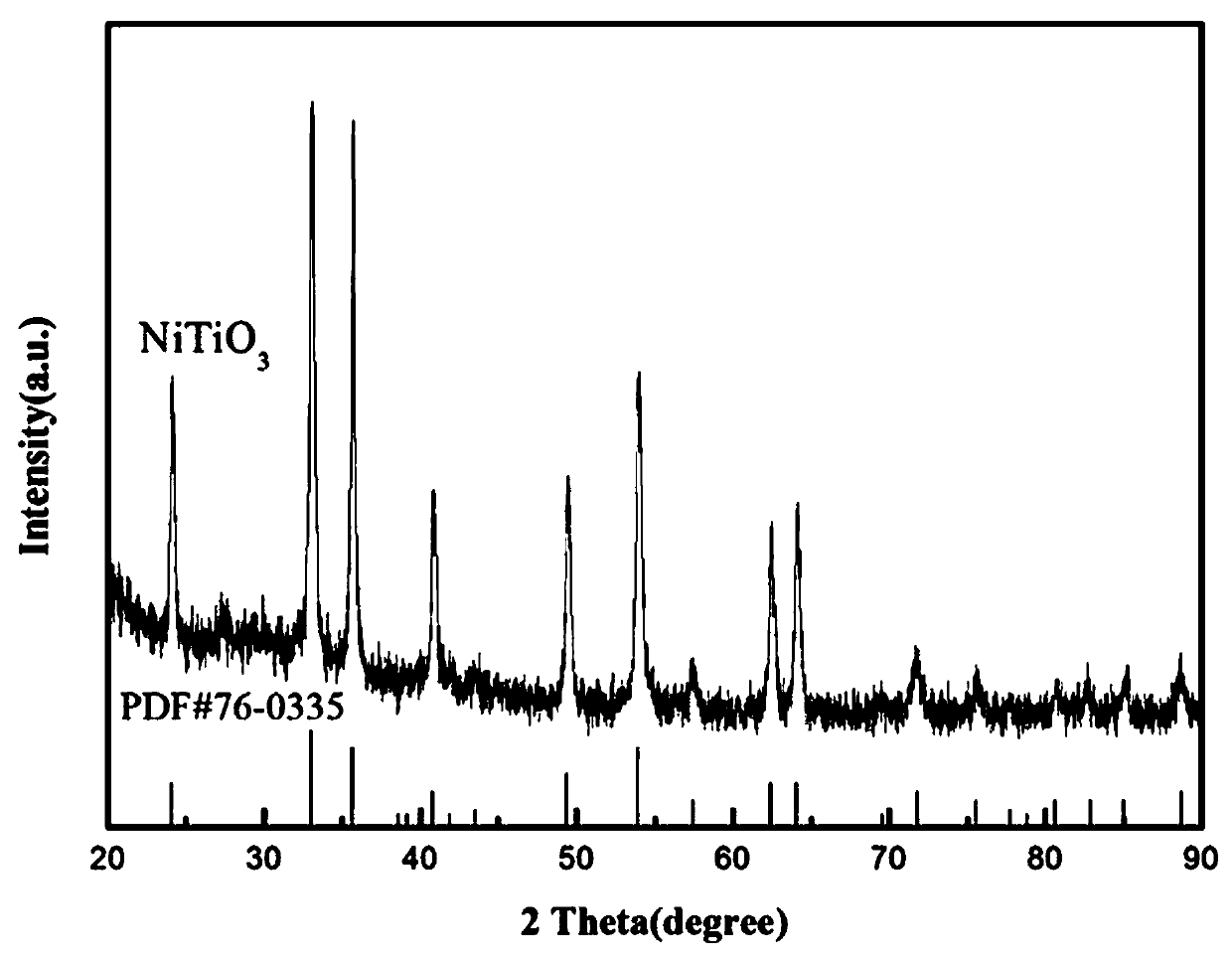
[0037] Step 1) Preparation of rod-shaped nickel titanate, with nickel chloride and butyl titanate at a ratio of 1:1, weigh 2.3769g of nickel chloride hexahydrate, add to 80 mL of ethylene glycol and stir to dissolve , then weighed 3.4 mL butyl titanate, slowly added to the above solution, and reacted for 3 h under the condition of 25 ℃ and 90 rpm / min magnetic stirring, and dissolved the obtained green suspension in absolute ethanol Centrifuge at 5000 rpm / min for 3 times, each centrifugation time is 10 min, and the centrifuged product is dried at 80 °C for 10 h under vacuum, and the dried product is put into a muffle furnace to raise the temperature to 600 °C at a rate of 5 °C / min. ℃, heat preservation for 1 hour to obtain rod-shaped nickel titanate;
[0038] Step 2) Nickel titanate doped lithium aluminum hydride hydrogen storage material (...
Embodiment 2
[0048] A nickel titanate doped lithium aluminum hydride hydrogen storage material (NiTiO 3 Content is the preparation method of 2 wt%), and the steps not specified in particular are the same as in Example 1, the difference is that: in the step 2, NiTiO 3 The addition amount of NiTiO was 2 wt%, and 0.0100 g NiTiO was weighed in an argon atmosphere glove box 3 and 0.4900 g LiAlH 4 .
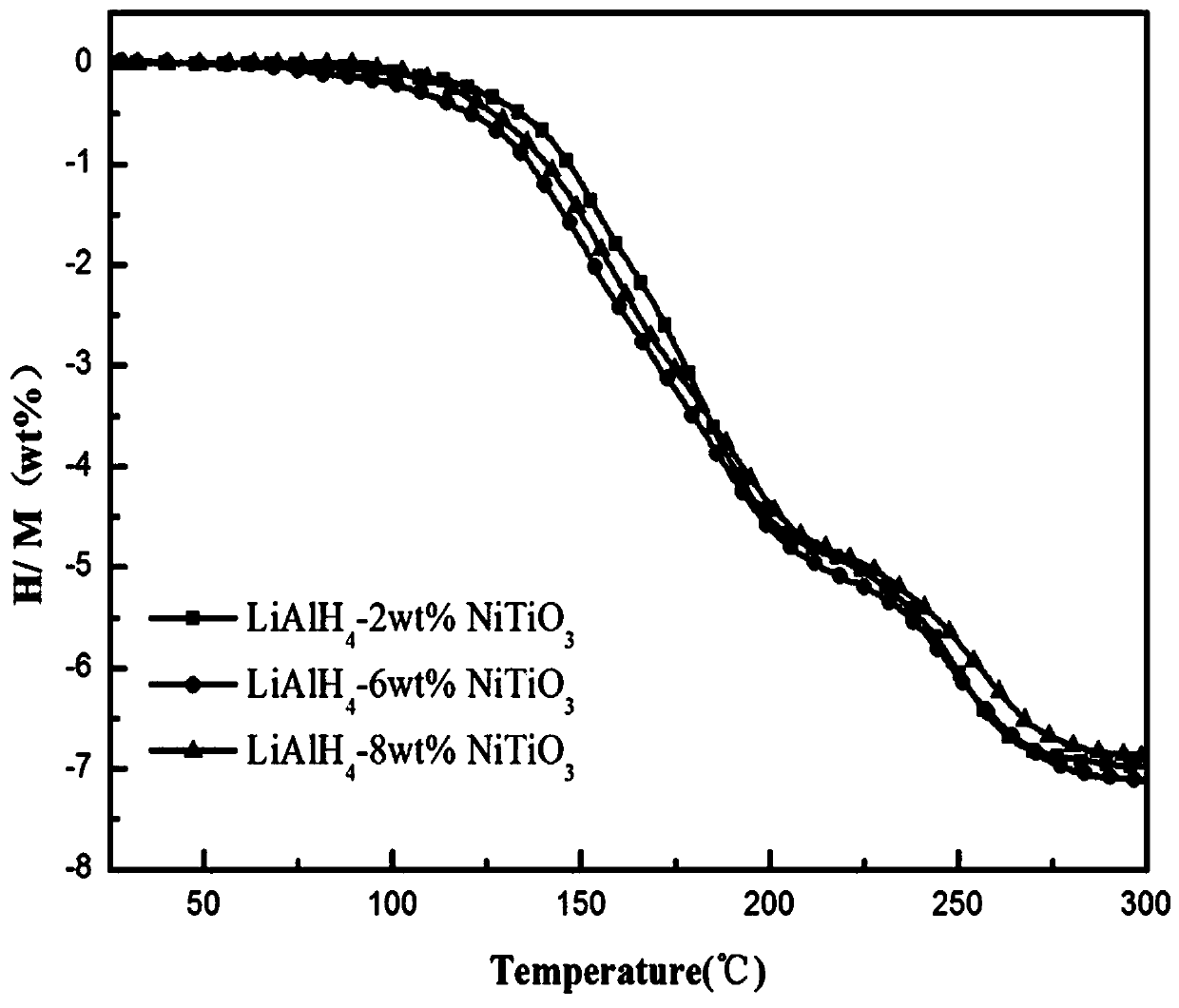
[0049] Will get NiTiO 3 The lithium aluminum hydride hydrogen storage material with a content of 2 wt% was subjected to a temperature-rising dehydrogenation test. The test method was the same as in Example 1, and the test results were as follows figure 2 As shown, the initial hydrogen desorption temperature is 95 ℃, and the hydrogen desorption amount is 7.0 wt% when the temperature rises to 300 ℃.
Embodiment 3
[0051] A nickel titanate doped lithium aluminum hydride hydrogen storage material (NiTiO 3 Content is the preparation method of 8 wt%), the step that does not specify in particular is identical with embodiment 1, difference is: in described step 2, NiTiO 3 The addition amount of NiTiO was 8 wt%, and 0.0400 g NiTiO was weighed in an argon atmosphere glove box 3 and 0.4600 g LiAlH 4 .
[0052] Will get NiTiO 3 The lithium aluminum hydride hydrogen storage material with a content of 8 wt% was subjected to a temperature-rising dehydrogenation test. The test method was the same as in Example 1, and the test results were as follows figure 2 As shown, the initial hydrogen desorption temperature is 98 ℃, and the hydrogen desorption amount is 6.9 wt% when the temperature rises to 300 ℃.
[0053] Therefore, NiTiO 3 The lithium aluminum hydride hydrogen storage material with a content of 6 wt% has the best hydrogen desorption performance. As shown in the figure, the initial hydrog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com