Carbon-loaded titanium dioxide-doped lithium aluminum hydride hydrogen storage material and preparation method thereof
A technology of titanium dioxide and lithium aluminum hydride, which is applied in the preparation/purification of titanium oxide/hydroxide, titanium dioxide, carbon, etc., can solve problems such as improvement, achieve low initial hydrogen release temperature, improve hydrogen release performance, and release hydrogen. The effect of hydrogen performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A method for preparing a carbon-supported titanium dioxide-doped lithium aluminum hydride hydrogen storage material, comprising the following steps:
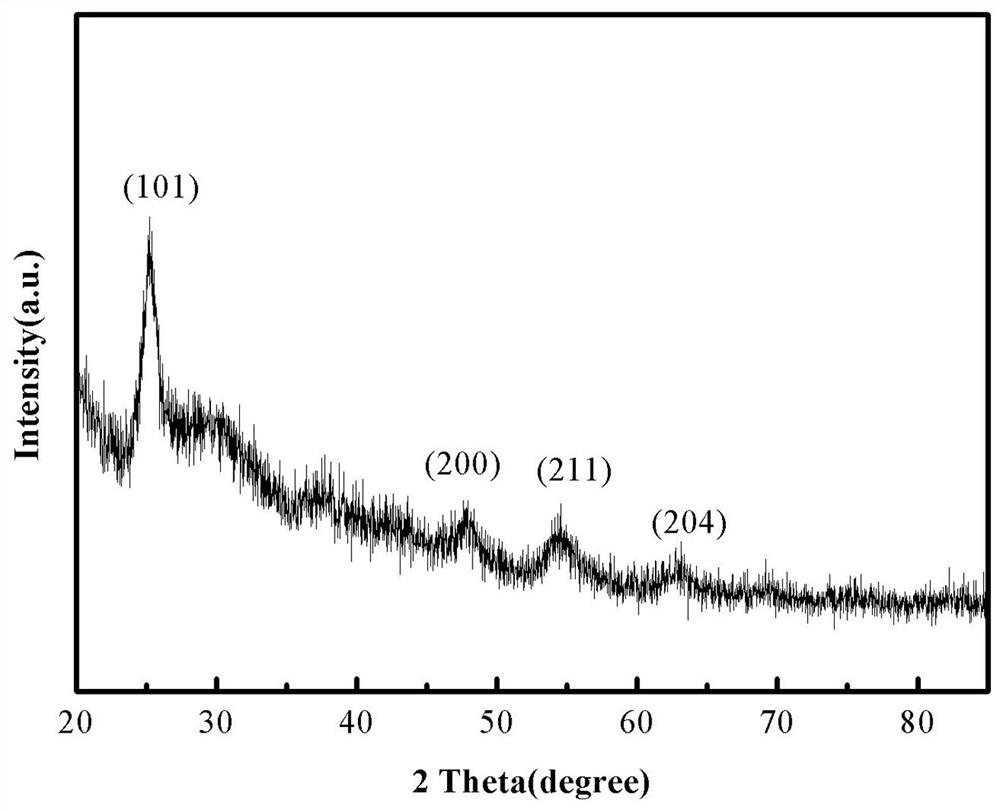
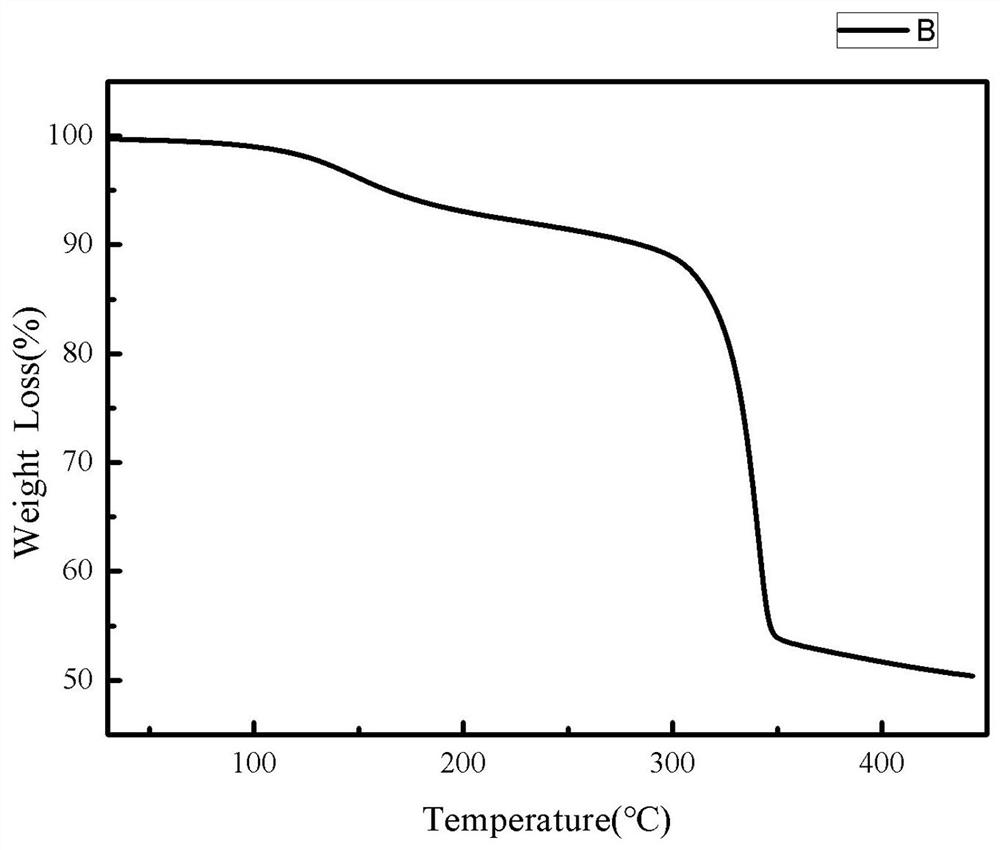
[0052] Step 1) Preparation of carbon-supported titanium dioxide generated in situ, measure glycerol, ethanol and butyl titanate at a volume ratio of 5:15:1, first mix glycerol and ethanol evenly as a solvent, and then , the solvent was magnetically stirred at a speed of 50 rpm / min, and butyl titanate was added dropwise to the above solvent while stirring, and the stirring time was 8 min. Then, the resulting mixed solvent was subjected to solvothermal reaction at 180 °C, and the reaction time was 24 h. After the reaction, the product was washed three times. To avoid hydrolysis, absolute ethanol was used as the detergent. The washed product was vacuum-dried at 60 °C for 12 h, and then the dried product was placed in a nitrogen atmosphere at 5 °C / Min heating rate increased to 450 °C, and the calcination time was 3 h, and th...
Embodiment 2
[0082] A carbon-supported titanium dioxide-doped lithium aluminum hydride hydrogen storage material (TiO 2 @C content is the preparation method of 2 wt%), the steps not specified in particular are the same as in Example 1, the difference is: in the step 2, TiO 2 The amount of @C added was 2 wt%, and 0.0100 g TiO was weighed in an argon atmosphere glove box 2 @C and 0.4900 g LiAlH 4 .
[0083] Will get TiO 2 @C content is 2 wt% Lithium aluminum hydride hydrogen storage material is subjected to temperature rise dehydrogenation test, the test method is the same as in Example 1, and the test results are as follows Figure 5 As shown, the initial hydrogen desorption temperature is 69 °C, and when the temperature rises to 300 °C, the hydrogen desorption amount is 7.36 wt%, and the hydrogen desorption rate reaches 97.4% of the theoretical value.
Embodiment 3
[0085] A carbon-supported titanium dioxide-doped lithium aluminum hydride hydrogen storage material (TiO 2 @C content is the preparation method of 8 wt%), the steps not specified in particular are the same as in Example 1, the difference is: in the step 2, TiO 2 The amount of @C added was 8 wt%, and 0.0400 g TiO was weighed in an argon atmosphere glove box 2 @C and 0.4600 g LiAlH 4 .
[0086] Will get TiO 2 @C content is 8 wt% lithium aluminum hydride hydrogen storage material to carry out temperature rise dehydrogenation test, test method is the same as embodiment 1, test result is as follows Figure 5 As shown, the initial hydrogen desorption temperature is 55 ℃, and when the temperature rises to 300 ℃, the hydrogen desorption amount is 6.89 wt%, and the hydrogen desorption rate reaches 97.1% of the theoretical value.
[0087] Therefore, TiO 2 The comprehensive hydrogen desorption performance of lithium aluminum hydride hydrogen storage materials with @C content of 6 wt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com