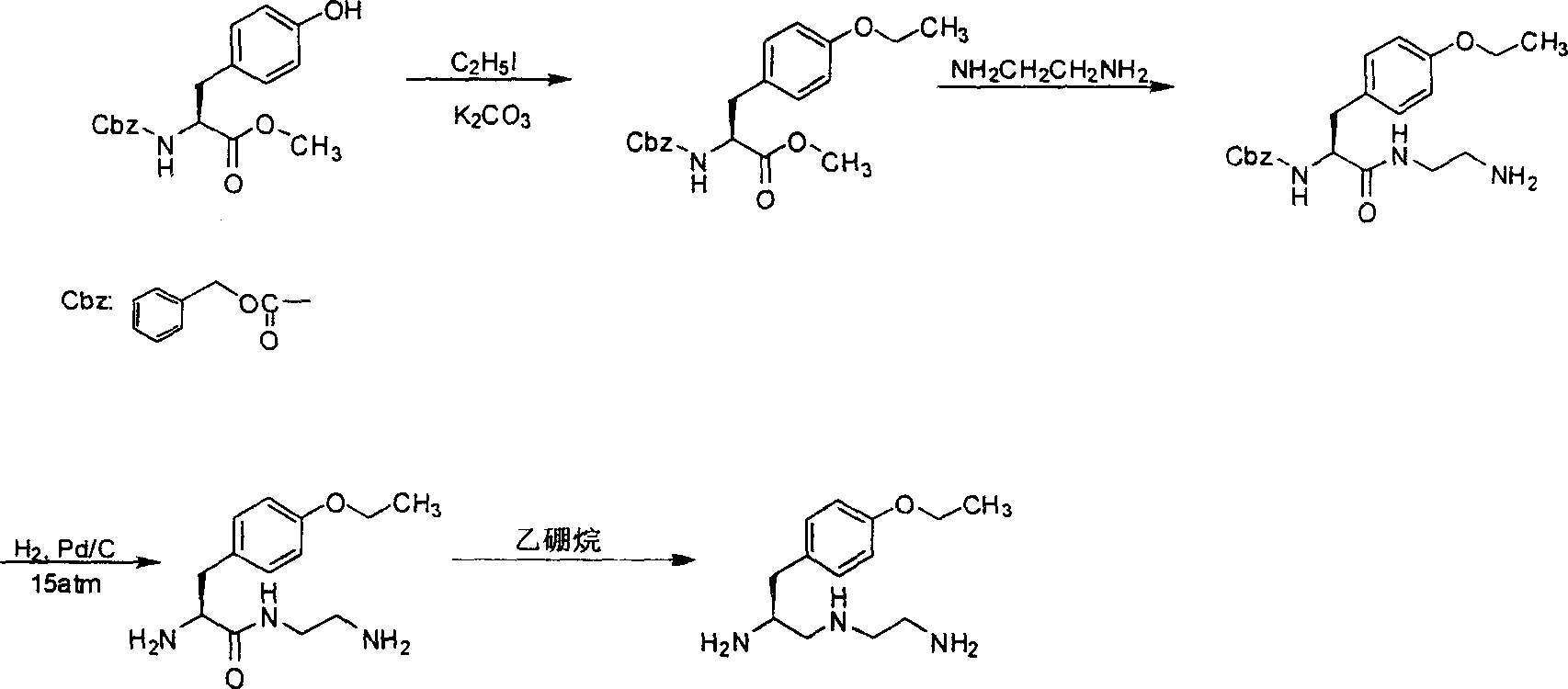
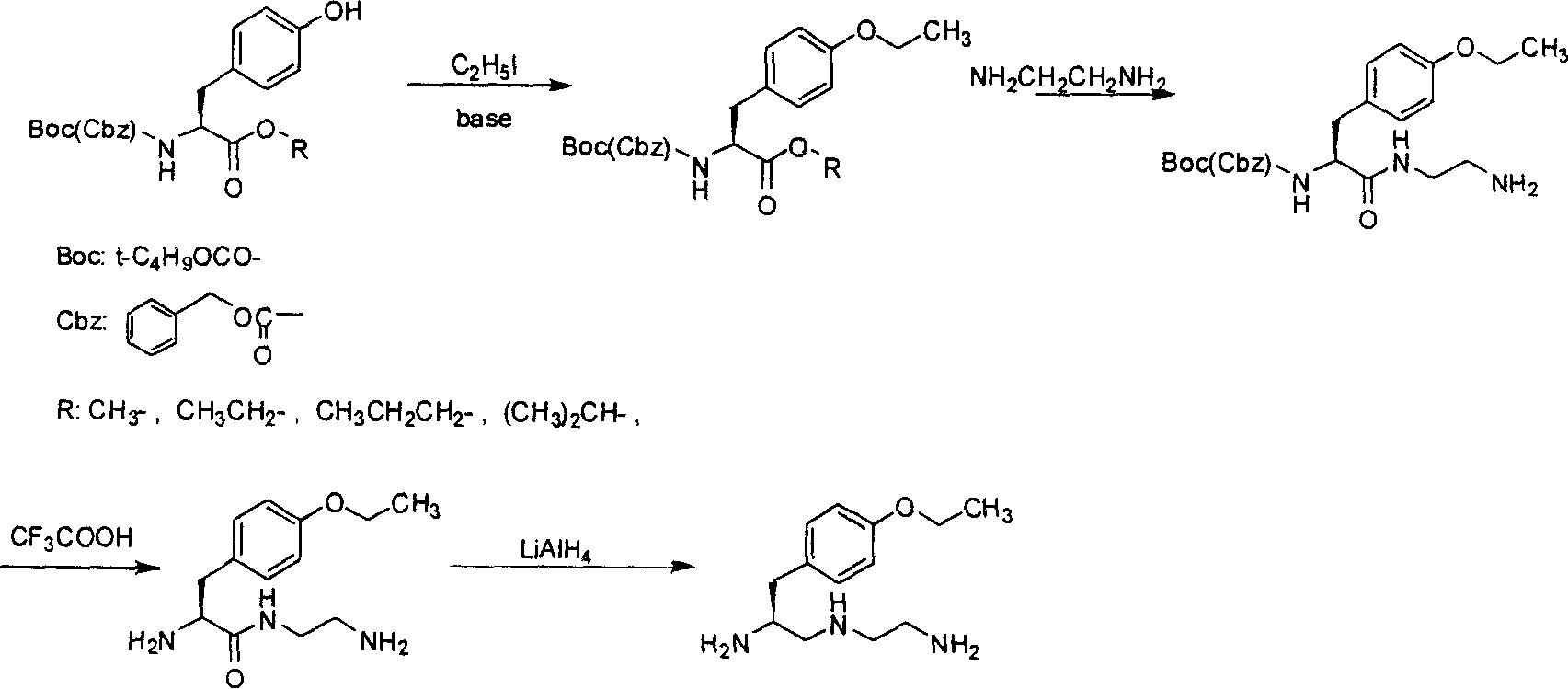
S-1-(4-ethoxybenzyl)-3-azapentane-1,5 diamine preparation method
An ethoxybenzyl and azapentane technology is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc. problem, to achieve the effect of high safety, short response time and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The synthesis of embodiment 1 O-ethyl-N-boc-L-tyrosine ethyl ester:
[0015] Add N-boc-L-tyrosine ethyl ester (24.8g, 0.08mol) and 180mL DMF into a 500mL three-necked flask, stir and dissolve, then add 22.0g of anhydrous potassium carbonate and iodoethane (7mL, 0.08mol), Keep stirring at room temperature at 25°C for 20 hours, extract with ethyl acetate for post-treatment, and evaporate to dryness under reduced pressure to obtain O-ethyl-N-boc-L-tyrosine ethyl ester (25.2g, 0.075mol), which is directly used for the following without purification One-step (embodiment 2) reaction, ethyl acetate is reclaimed and used. The molar yield was 93.8% (the content detected by HPLC was 96.2%).
Embodiment 2
[0016] Example 2 Synthesis of N-(2-aminoethyl)-N-boc-O-ethyl-L-tyrosinamide:
[0017] Dissolve the O-ethyl-N-boc-L-tyrosine ethyl ester (25.2 g, 0.075 mol) obtained in the previous step (Example 1) in 100 mL of methanol, and slowly add it dropwise until anhydrous ethylenediamine is added (90g, 1.5mol) in a 500mL three-necked flask, after the dropwise addition was completed, the stirring was continued at 50°C for 20 hours. Evaporate to dryness under reduced pressure, add 60 mL of ethyl acetate to the residue, heat to dissolve and let stand, collect the precipitated white solid, and dry at 50°C to obtain N-(2-aminoethyl)-N-boc-O-ethyl- L-tyrosinamide (20 g, 0.054 mol), the molar yield was 72%, and ethyl acetate was recovered for use. mp 94-96°C, MS: 367.
Embodiment 3
[0018] Example 3 Synthesis of N-(2-aminoethyl)-O-ethyl-L-tyrosinamide:
[0019] Add N-(2-aminoethyl)-N-boc-O-ethyl-L-tyrosinamide (32g, 0.087mol) and 400mL dichloromethane into a 500mL three-necked flask, stir and dissolve, then add trifluoroacetic acid ( 22.8g, 0.2mol) was stirred at room temperature for 1 hour, concentrated and evaporated to dryness under reduced pressure to obtain N-(2-aminoethyl)-O-ethyl-L-tyrosyl (18g, 0.072mol), the molar yield was 83% , mp94~96℃ (refined product), MS: 251.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com