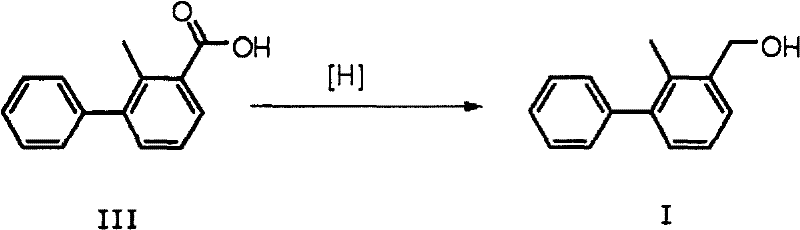
Preparation method of 2-methyl-3-biphenylmethanol
A technology of phenyl benzyl alcohol and methyl benzoic acid is applied in the field of preparation of 2-methyl-3-phenyl benzyl alcohol, can solve the problems of harsh reaction conditions, low yield, many side reactions, etc., and achieves the reaction yield. The effect of high rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
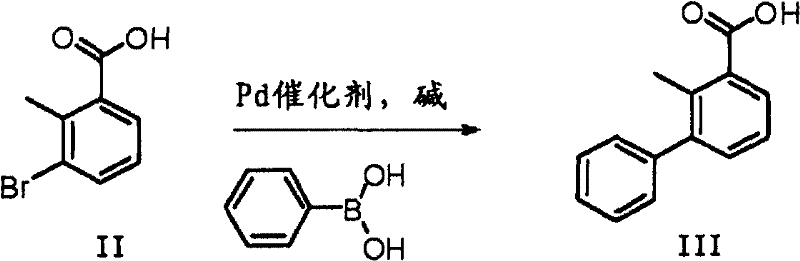
[0030] Add 3-bromo-2-methylbenzoic acid (4.9g, 22.4mmol), palladium acetate (55mg, 0.23mmol), sodium carbonate (7.2g, 68.1mmol) to the 500mL reaction flask under nitrogen protection (reaction system pH 8-9), phenylboronic acid (3.1 g, 25 mmol) and water (125 mL). The reaction mixture was stirred at room temperature for 1 hour. The reaction mixture was poured into 2N hydrochloric acid, the product was extracted with ethyl acetate (3×150 mL), the organic layers were combined, dried and concentrated to give the title compound (90% yield).
Embodiment 2
[0032] Add 3-bromo-2-methylbenzoic acid (4.9g, 22.4mmol), 5% palladium / carbon (1.12mmol), potassium carbonate (6.2g, 44.8mmol) to the 500mL reaction flask under nitrogen protection (reaction system pH 8-9), phenylboronic acid (3.1 g, 25 mmol) and a mixture of N,N-dimethylacetamide and water (20 / 1 (v / v), 125 mL). The reaction mixture was stirred at 80 °C for 1 hour. The reaction mixture was poured into 2N hydrochloric acid, the product was extracted with ethyl acetate (3×150 mL), the organic layers were combined, dried and concentrated to give the title compound (94% yield).
Embodiment 3
[0034] Add 3-bromo-2-methylbenzoic acid (4.9g, 22.4mmol), palladium / carbon (1.12mmol), sodium carbonate (4.75g, 44.8mmol) in 500mL reaction flask (reaction system pH value is 8-9 ), sodium tetraphenylborate (2.0 g, 6.05 mmol) and water (125 mL). The reaction mixture was stirred and refluxed for 3 hours. The reaction mixture was poured into 2N hydrochloric acid, the product was extracted with ethyl acetate (3×150 mL), the organic layers were combined, dried and concentrated to give the title compound (92% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com