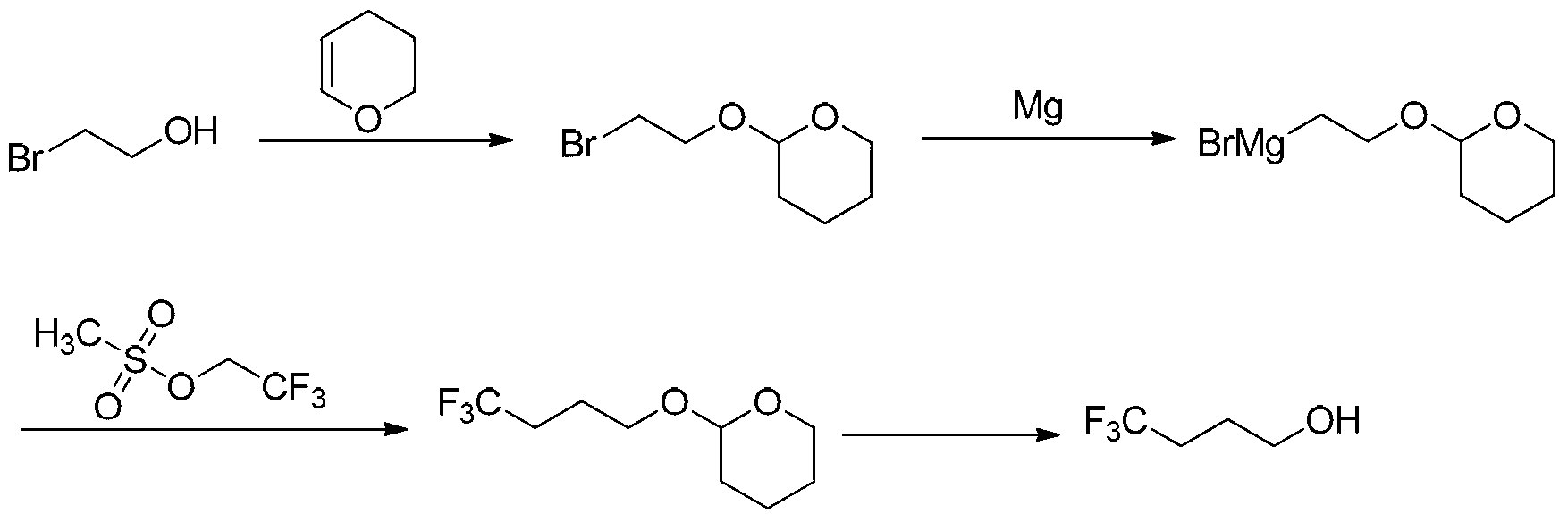
Method synthesizing 4, 4, 4-trifluoro butanol
A technology of trifluorobutanol and a synthesis method, which is applied in the directions of hydrolysis preparation, organic chemistry, etc., can solve the problems of low yield and high price of lithium aluminum hydride, and achieve the effects of short process route, favorable industrial production and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthetic steps of 4,4,4-trifluorobutanol are as follows:
[0041]Step 1: Add 200g (1.6mol) 2-bromoethanol, 12.0g (0.048mol) pyridinium p-toluenesulfonate and 400mL dichloromethane into a 1000mL three-necked flask, add 201.6g ( 2.4mol) 3,4-dihydropyran, keep the temperature at 20-30°C and continue the reaction for 1 hour after the dropwise addition. After the reaction of the raw material 2-bromoethanol is completed by gas chromatography, add 200mL water to the system, and let it stand for separation. liquid, the organic phase was washed twice with water, dried over anhydrous magnesium sulfate, the desiccant was filtered off, the solvent was evaporated by a rotary evaporator under reduced pressure, the remaining liquid was distilled under reduced pressure, and fractions at 98-102°C were collected (at a pressure of 3000Pa) to obtain 301.2 g of a transparent liquid, namely the 2-(2-bromoethoxy)tetrahydro-2H-pyran, with a gas chromatography content of 98.9% and a yield ...
Embodiment 2
[0049] The preparation process is the same as in Example 1, except that:
[0050] The catalyst in step 1 is p-toluenesulfonic acid, the molar ratio of 2-bromoethanol to 3,4-dihydropyran is 1:3, and the yield in step 1 is 89.6%;
[0051] In step 2, the molar ratio of 2-(2-bromoethoxy)tetrahydro-2H-pyran to magnesium powder is 1:2;
[0052] The catalyst in step 3 is dilithium tetrachloroketone, and the catalyst dosage is 5% of the molar weight of 2,2,2-trifluoroethyl methanesulfonate, and the yield in step 3 is 54.6%;
[0053] The catalyst in step 4 is Dowex-50 (H type) cation exchange resin, and the catalyst dosage is 1 to 10 based on the mass of 2-(4,4,4-trifluorobutoxy)tetrahydro-2H-pyran %, preferably 10%, step 4 yield is 65%, product gas chromatography content 98.5%.
Embodiment 3
[0055] The preparation process is the same as in Example 1, except that:
[0056] In step 1, the molar ratio of 2-bromoethanol to 3,4-dihydropyran is 1:1, and the yield in step 1 is 86.2%;
[0057] In step 2, the molar ratio of 2-(2-bromoethoxy)tetrahydro-2H-pyran to magnesium powder is 1:1;
[0058] The catalyst in step 3 is dilithium tetrachloroketone, and the catalyst dosage is 1% of the molar weight of 2,2,2-trifluoroethyl methanesulfonate, and the yield in step 3 is 50.1%;
[0059] The solvent in step 4 is methanol, the catalyst is Dowex-50 (H type) cation exchange resin, and the amount of catalyst is based on the mass of 2-(4,4,4-trifluorobutoxy)tetrahydro-2H-pyran 1%, step 4 yield is 60%, product gas chromatography content 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com