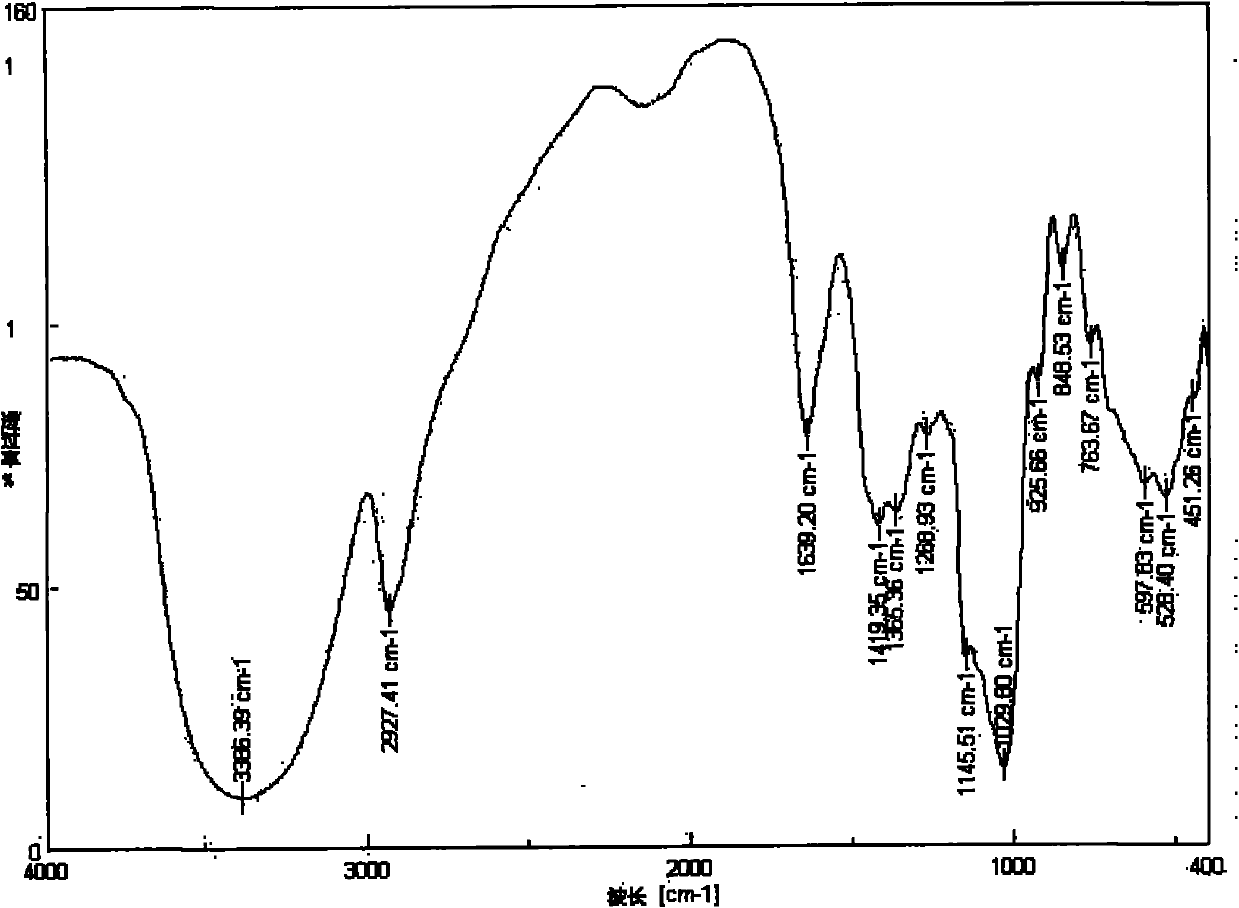
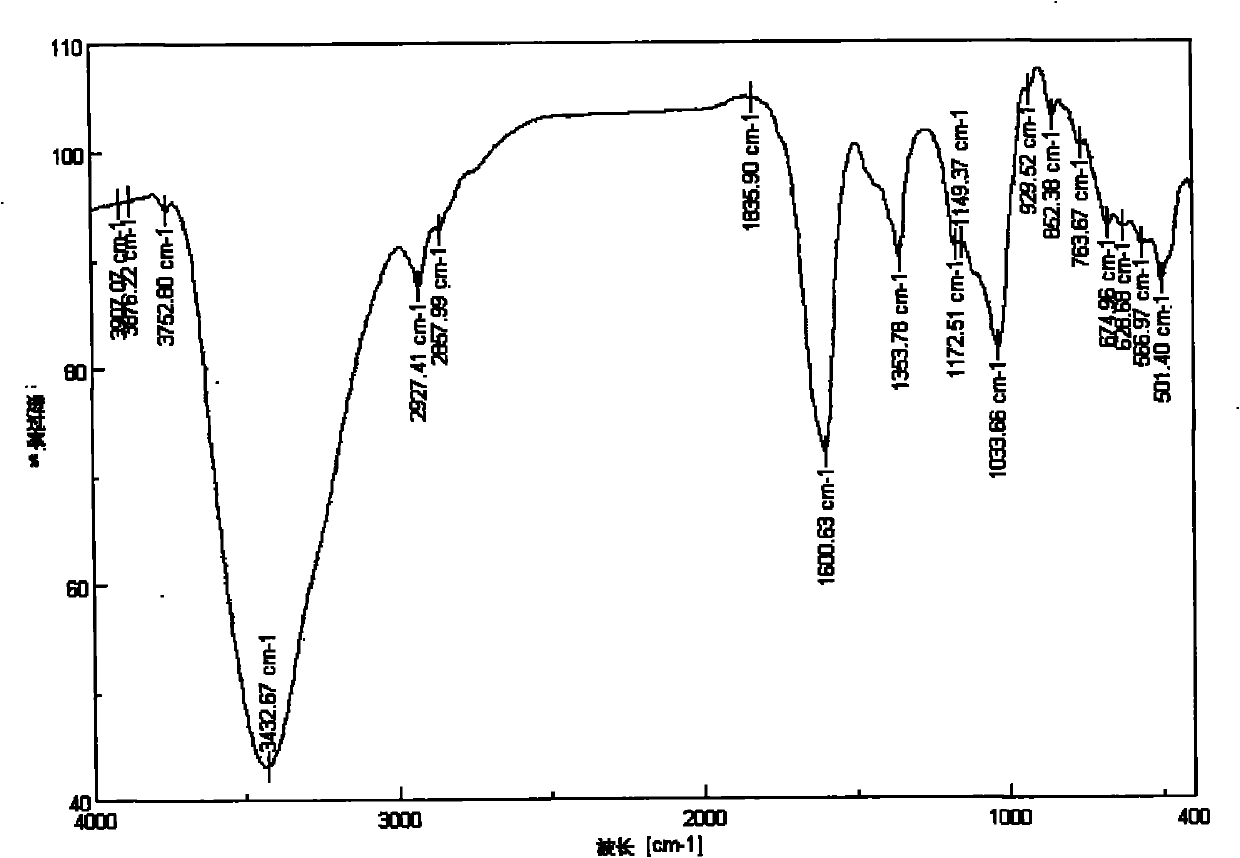
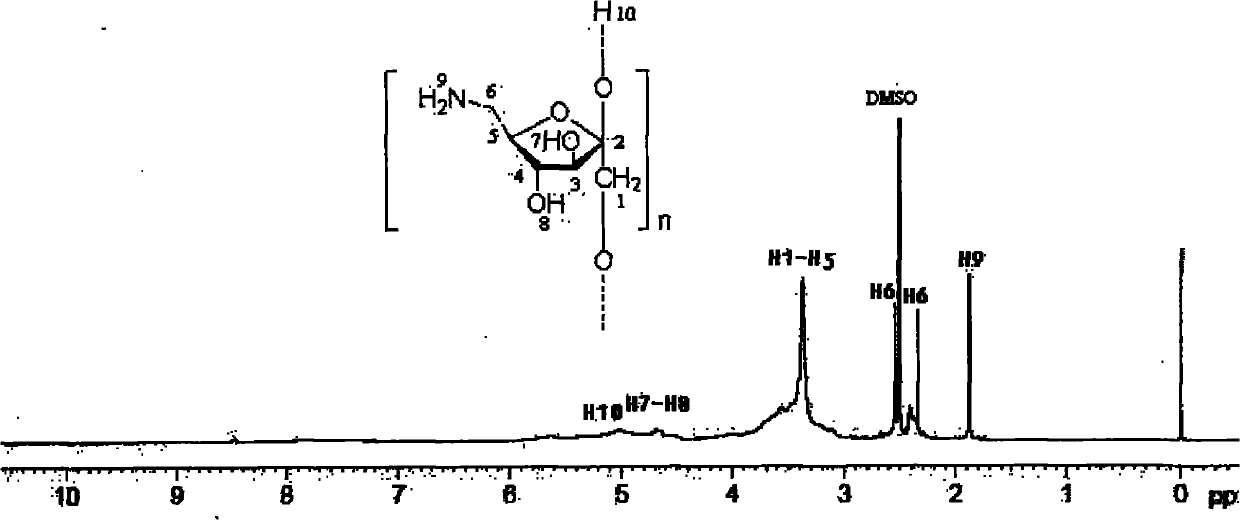
6-amino-6-deoxyinulin as well as preparation and application thereof
A technology of deoxyinulin and amino group is applied in the field of daily chemicals, which can solve the problems of less renewable resources and the like, and achieve the effects of easy popularization, improved reactivity and simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 6-Amino-6-deoxyinulin is a compound represented by formula (1).
[0028] Formula 1)
[0029] (1) Take inulin for sulfonyl chloride esterification, specifically, dissolve 1.62 g of inulin and 1.90 g of p-toluenesulfonyl chloride into 30 mL of nitrogen-nitrogen dimethylformamide, and stir and react at -8°C for 10 h. After the reaction was finished, the reaction solution was poured into 150 mL of acetone, and a precipitate was precipitated. The precipitate was washed with acetone and tetrahydrofuran, then vacuum freeze-dried, and set aside.
[0030] The prepared product was p-tosylated inulin (3.2 g) dissolved in 20 mL of dimethyl sulfoxide and reacted with 1.3 g of sodium azide at 50° C. for 8 h. After the reaction was completed, the product was precipitated with 100 mL of acetone, washed with ether and acetone, respectively, and then vacuum-dried at -50°C to obtain 1.4 g of 6-azido-6-deoxyinulin, which was used for future use.
[0031] (2) Dissolve 1.4 g of the prod...
Embodiment 2
[0037] The difference from Example 1 is:
[0038] (1) Take inulin for halogenation reaction, and dry inulin and lithium bromide under vacuum at 100° C. overnight. Take 1.62g of inulin and 1g of lithium bromide and add them to 20mL of purified nitrogen-nitrogen dimethylformamide under the protection of nitrogen, raise the temperature until inulin and lithium bromide are completely dissolved, and add 3.5g of N- Bromosuccinimide (NBS) was added to the above reaction solution. Weigh 5.2 g of triphenylphosphine and dissolve it in 10 mL of nitrogen-nitrogen dimethylformamide. This solution was added dropwise to the reaction solution at room temperature. After the reaction solution was reacted at room temperature for 30 minutes, the temperature of the reaction system was raised to 70°C. After the reaction was carried out at 70°C for 3 hours, the reaction solution was poured into 150mL acetone, and a precipitate was precipitated. The precipitate was suction filtered, washed with ace...
Embodiment 3
[0042] The difference from Example 1 is that: 1.62g of inulin and 1.90g of p-toluenesulfonyl chloride were dissolved in 30mL of nitrogen-nitrogen dimethylformamide, and stirred and reacted at 5°C for 16 hours. After the reaction was finished, the reaction solution was poured into 150 mL of acetone, and a precipitate was precipitated. The precipitate was washed with acetone and tetrahydrofuran, and then vacuum freeze-dried to obtain 3.1 g of tosylated inulin.
[0043] The product obtained above was dissolved and then reacted with 1.0 g of sodium azide at 50° C. for 16 h. After the reaction, the product was precipitated with 100 mL of acetone, washed with ether and acetone, respectively, and vacuum-dried at -50°C to obtain 1.5 g of 6-azido-6-deoxyinulin, which was ready for use.
[0044] 1.5 g of the obtained 6-azido-6-deoxyinulin and 0.6 g of lithium aluminum hydride were dissolved in 20 mL of purified 1,4-dioxane, and reacted at 55° C. for 19 h under nitrogen protection. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com