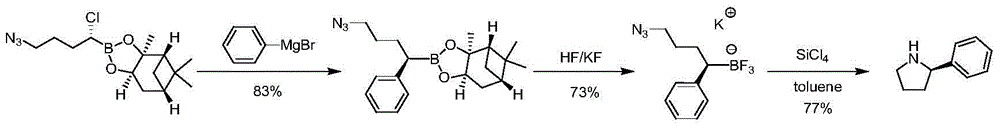
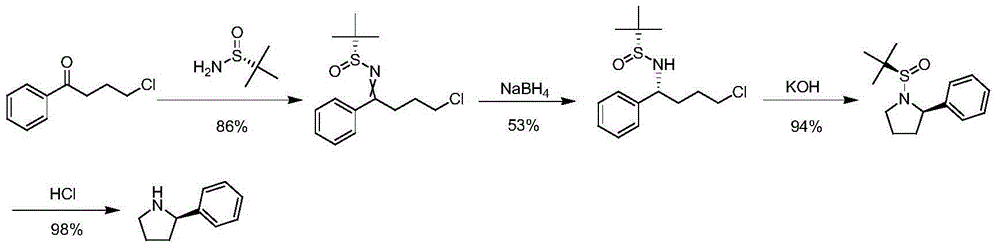
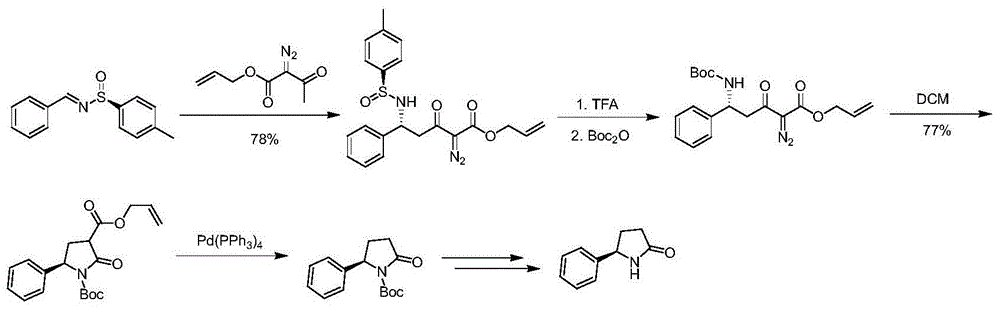
Synthetic method of chiral 2-phenylpyrrolidine
The technology of a phenylpyrrolidine and a synthetic method is applied in the synthesis of chiral 2-phenylpyrrolidine and the synthesis field of chiral pyrrolidine, and can solve the problems of being unsuitable for industrial production, expensive reagents or catalysts, and difficult to obtain, etc., Achieve the effect of low cost, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of (S)-2-phenylpyrrolidine:
[0041] (1) Dissolve (R)-2-amino-2-phenylacetic acid (200g, 1.32mol), potassium carbonate (218g, 1.58mol) in water (2.0L) and methanol (0.5L), cool to T <10°C, add di-tert-butyl dicarbonate (288g, 1.32mol) dropwise, react overnight at room temperature, adjust pH to 4-5 with 1N HCl, extract with ethyl acetate (1.0L*3), and saturated saline (1.0 L*1) was washed and concentrated to obtain 315 g of white solid with a yield of 95%.
[0042] h 1 NMR (400MHz, DMSO-d6): 12.74(s, 1H), 7.55(d, J=8Hz, 1H), 7.38-7.3(m, 5H), 5.10(d, J=8Hz, 1H), 1.39(s ,9H).
[0043] (2) Add (R)-2-(tert-butoxycarbonylamino)-2-phenylacetic acid (300g, 1.19mol), 4-dimethylaminopyridine (260g, 2.12mol), cycloisopropylidene malonate The ester (205g, 1.43mol) was dissolved in dichloromethane (3.0L), and N,N-dicyclohexylcarbodiimide (370g, 1.79mol) was dissolved in dichloromethane (0.5L), keeping the temperature at Drop into the reaction system ...
Embodiment 2
[0049] Embodiment 2: the synthesis of (R)-2-phenylpyrrolidine:
[0050] (1) Dissolve (S)-2-amino-2-phenylacetic acid (100g, 0.66mol), potassium carbonate (109g, 0.79mol) in water (1.0L) and methanol (0.25L), cool to T <10°C, add di-tert-butyl dicarbonate (144g, 0.66mol) dropwise, react overnight at room temperature, adjust pH to 4-5 with 1N HCl, extract with ethyl acetate (0.5L*3), and saturated saline (0.5 L*1) was washed and concentrated to obtain 150 g of white solid with a yield of 90%.
[0051] h 1 NMR (400MHz, DMSO-d6): 12.72(s, 1H), 7.53(d, J=8Hz, 1H), 7.38-7.3(m, 5H), 5.09(d, J=8Hz, 1H), 1.38(s ,9H).
[0052] (2) (S)-2-(tert-butoxycarbonylamino)-2-phenylacetic acid (150g, 0.59mol), 4-dimethylaminopyridine (130g, 1.06mol), cycloisopropylidene malonate Ester (102g, 0.71mol) was dissolved in dichloromethane (1.5L), and N,N-dicyclohexylcarbodiimide (185g, 0.89mol) was dissolved in dichloromethane (0.25L), keeping the temperature at Drop into the reaction system at 0-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com