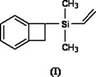
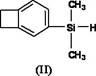
Silicon-containing benzocyclobutene monomers and preparation method thereof
A technology of bromobenzocyclobutene and dimethylbenzocyclobutene is applied in the field of silicon-containing benzocyclobutene monomer and its preparation, and can solve problems such as poor film-forming performance, and achieve faster reaction speed, The effect of reducing side reactions and excellent overall performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of 1-dimethylvinylsilyl benzocyclobutene:
[0034] Vacuumize the vial containing the magnetic stirrer and fill it with nitrogen three times, then in nitrogen (N 2 ) under the protection of magnesium (Mg) (264mg, 11mmol), anhydrous lithium chloride (LiCl) (467.5mg, 11mmol), lithium aluminum hydride (LiAlH 4 ) (76mg, 2mmol) was added to the vial and stirred by magnetic force for a period of time. A solution of 1-BrBCB (1-bromobenzocyclobutene) (1.3 mL, 11 mmol) in tetrahydrofuran (26 mL) was added under nitrogen. Stir rapidly at room temperature for 2 hours. After the magnesium strips are almost completely reacted, add a solution of dimethylvinylchlorosilane (1.4 mL, 10 mmol) in tetrahydrofuran (5 mL) dropwise, and stir rapidly at room temperature for 1 hour. Add a certain amount of water to stop the reaction, extract with ether, combine the organic phases, and use anhydrous MgSO 4 Dry it, let it stand overnight, filter it with suction, and separ...
Embodiment 2
[0036] The preparation method of 1-dimethylvinylsilyl benzocyclobutene:
[0037] Vacuumize the vial containing the magnetic stirrer and fill it with nitrogen three times, then in nitrogen (N 2 ) under the protection of magnesium strips (2.64g, 110mmol), anhydrous lithium chloride (LiCl) (467.5mg, 110mmol) and lithium aluminum hydride (LiAlH 4 ) (760mg, 20mmol) was added to the vial, and magnetically stirred for a period of time. A solution of 1-BrBCB (1-bromobenzocyclobutene) (13 mL, 110 mmol) in tetrahydrofuran (260 mL) was added under nitrogen. Stir rapidly at 30°C for 5 hours, after the magnesium strips are almost completely reacted, add dropwise a solution of dimethylvinylchlorosilane (14mL, 100mmol) in tetrahydrofuran (50mL), and stir rapidly at 30°C for 3 hours. Add a certain amount of water to stop the reaction, extract with ether, combine the organic phases, and use anhydrous MgSO 4 Dry it, let it stand overnight, filter it with suction, and separate and purify it b...
Embodiment 3
[0039] The preparation method of 1-dimethylvinylsilyl benzocyclobutene:
[0040] Vacuumize the vial containing the magnetic stirrer and fill it with nitrogen three times, then in nitrogen (N 2 ) under the protection of magnesium strips (26.4mg, 1.1mmol), anhydrous lithium chloride (LiCl) (46.7mg, 0.126 mmol) and lithium aluminum hydride (LiAlH 4 ) (7.6mg, 0.2mmol) was added to the vial and stirred by magnetic force for a period of time. A solution of 1-BrBCB (1-bromobenzocyclobutene) (0.13 mL, 1.1 mmol) in tetrahydrofuran (0.4 mL) was added under nitrogen. Stir rapidly at room temperature for 2 hours. After the magnesium strips are almost completely reacted, add a solution of dimethylvinylchlorosilane (0.14 mL, 1 mmol) in tetrahydrofuran (1 mL) dropwise, and stir rapidly at room temperature for 1 hour. Add a certain amount of water to stop the reaction, extract with ether, combine the organic phases, and use anhydrous MgSO 4 Dry it, let it stand overnight, filter it with su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com