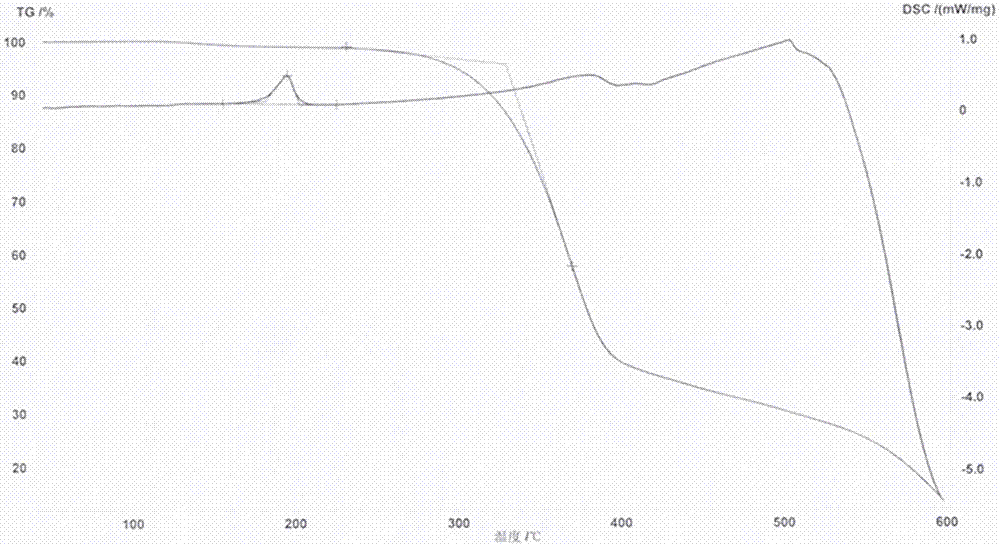
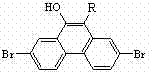
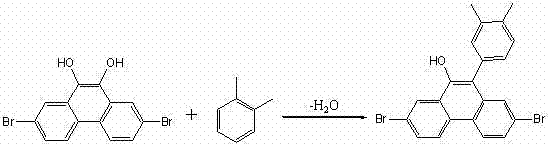
2,7-dibromo-9-hydroxyl phenanthrene derivatives and preparation method thereof
A technology of hydroxyphenanthrene derivatives, which is applied in the field of 2,phenanthrene derivatives and their preparation, can solve problems such as light blue light emission and complex synthesis of bicyclic systems, and achieve improved solubility, easy availability of raw materials, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1: Synthesis of 2,7-dibromophenanthrenequinone
[0020]
[0021] Under full stirring, 17.95 g NBS was added to 98% concentrated sulfuric acid containing 10 g 9,10-diphenanthrenequinone, and the stirring was continued for 3 h at room temperature, then the mixture was poured on ice cubes, the organic matter was filtered, washed with cold water, and the crude product Recrystallized with DMSO to obtain 13 g orange-red 2,7-dibromophenanthrenequinone, yield: 75%.
[0022] 1 H NMR (300 MHz, DMSO), δ / ppm: 8.30-8.21 (2H), 8.13-8.04 (2H), 8.03-7.81 (2H).
[0023] Ms ( m / z ): 366 (M + )
example 2
[0024] Example 2: Synthesis of 2,7-dibromo9,10-dihydroxyphenanthrene
[0025]
[0026] Under reflux, add 10 mL of concentrated hydrochloric acid in batches to 50 mL of glacial acetic acid solution containing 3.66 g of 2,7-dibromophenanthrenequinone and 4 g of Sn, continue to reflux for 1 h, evaporate most of the glacial acetic acid, and pour the rest Pour into water, filter, wash with water, and dry to obtain 3.50 g flesh red 2,7-dibromo9-10-dihydroxyphenanthrene, with a purity greater than 98% and a yield of 96%.
[0027] 1 H NMR (300 MHz, CDCl 3 ) , δ / ppm: 8.32-8.31 ( 2H ), 7.85-7.84 ( 4H ).
[0028] Ms ( m / z ): 368 (M + ).
example 3
[0029] Example 3: Synthesis of 2,7-dibromo-9-hydroxyl-10-p-tolylphenanthrene
[0030]
[0031] Add 3.70 g of 2,7-dibromo9-10-dihydroxyphenanthrene and a catalytic amount of trifluoromethanesulfonic acid to 20 mL of toluene as a solvent, heat to reflux for 2 h, wash the reaction mixture with water, evaporate the solvent, add an appropriate amount of THF, and filter. The THF was distilled off to give 3.98 g of a gray solid. The purity is greater than 98%, and the yield is 90%.
[0032] 1 H NMR (300 MHz, CDCl 3 ), δ / ppm: 8.51-8.50 ( 1H ), 8.48-8.41 ( 2H ), 7.78-7.75 ( 1H ), 7.57-7.54 ( 1H ), 7.52-7.51 ( 1H ), 7.46-7.43 ( 2H ), 7.31- 7.29 ( 2H ), 5.55 ( 1H ), 2.50 ( 3H ).
[0033] Ms(m / z): 442 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com