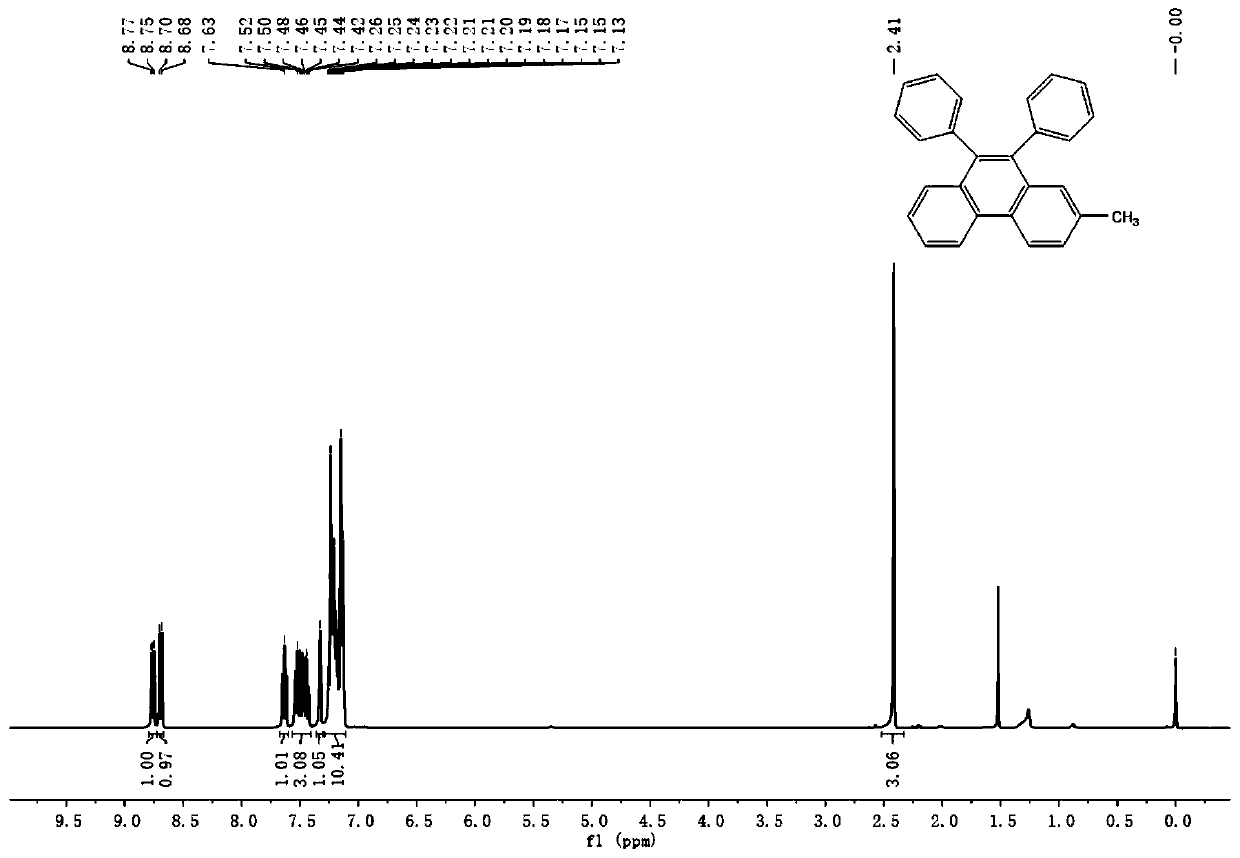
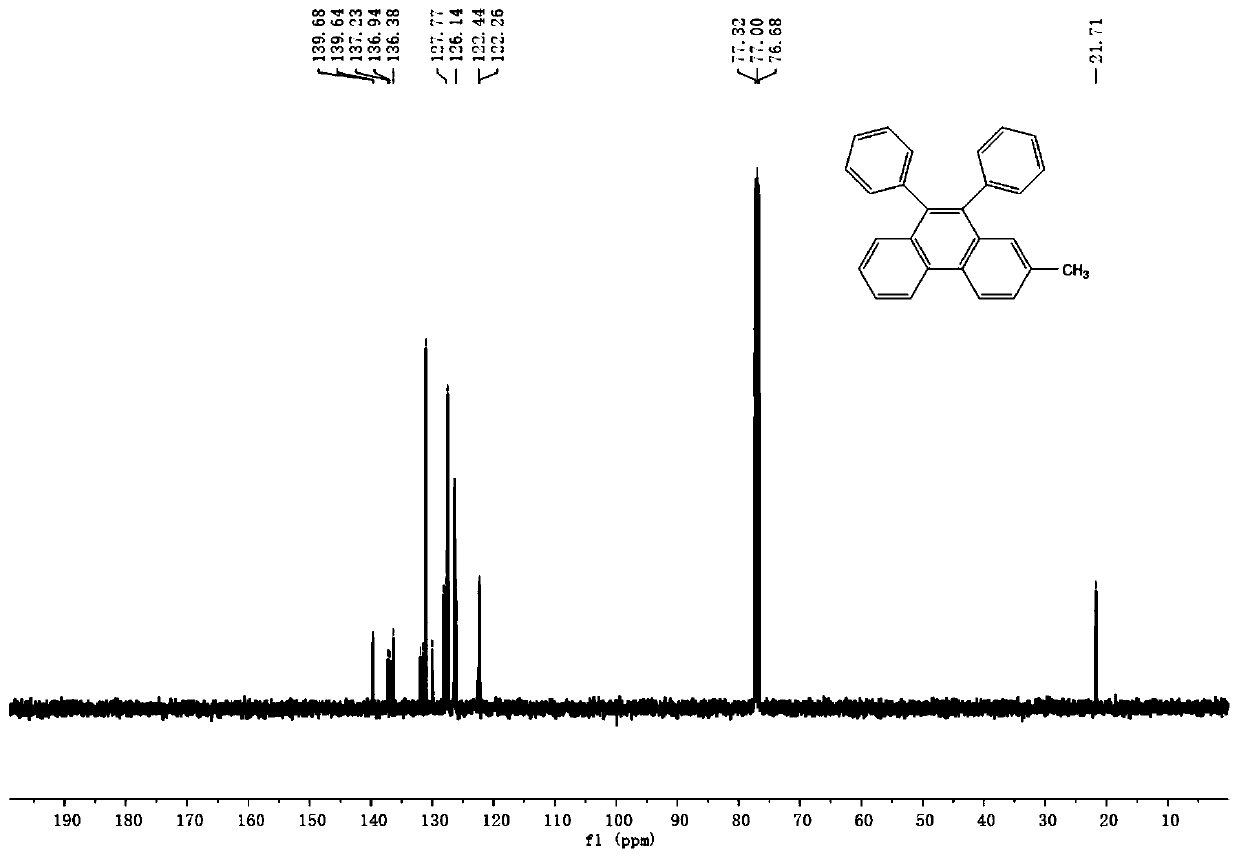
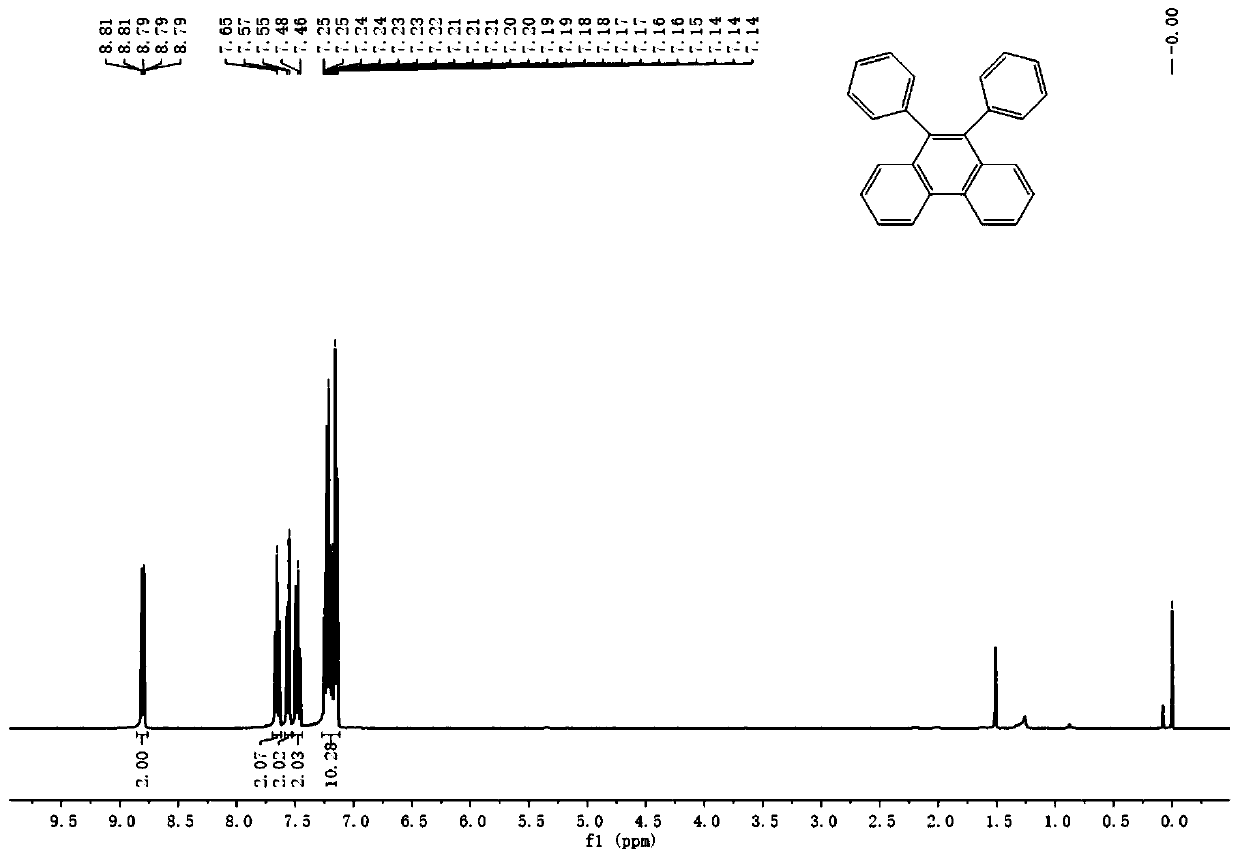
Synthetic method of phenanthrene, and phenanthrene derivative
A synthesis method and technology of derivatives, which are applied in chemical instruments and methods, preparation of organic compounds, hydrocarbon compounds, etc., to achieve the effects of mild reaction conditions, simple preparation methods, and good scalability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Add 0.015mmol of palladium acetate, 0.03mmol of bis(2-diphenylphosphophenyl)ether, 0.6mmol of cesium carbonate, 0.3mmol of pivalic acid, 0.3mmol of 4-methylphenylboronic acid, and 0.75mmol of toluene into the dry Schlenk tube, replace the gas atmosphere in the Schlenk tube from air to nitrogen, and replace the system three times in a nitrogen atmosphere of a standard atmospheric pressure to ensure that the system has a pure nitrogen atmosphere; under a nitrogen atmosphere, add 0.3mmol o-bromoiodobenzene and 2mL Heat toluene to 120°C, stir for 24 hours, and cool to room temperature; add 4 mL of saturated ammonium chloride solution to quench the reaction, add 8 mL of water, extract with ethyl acetate, separate and purify by column chromatography to obtain the tolan derivative, The isolated yield of this example reaches 92%.
Embodiment 2-6
[0065] The difference with Example 1 is that the arylboronic acids added in Examples 2-6 are respectively phenylboronic acid, 4-tert-butylphenylboronic acid, 4-butylphenylboronic acid, 4-methoxyphenylboronic acid, 4 -Fluorophenylboronic acid, other preparation steps remain unchanged. The reactant and productive rate of embodiment 1-6 are as table 1.
[0066] The general reaction formula of embodiment 1-6 is:
[0067]
[0068] Table 1 Reaction of o-bromoiodobenzene, tolanyne and different arylboronic acids
[0069]
Embodiment 7
[0071] Add 0.015mmol of palladium acetate, 0.03mmol of bis(2-diphenylphosphophenyl)ether, 0.6mmol of cesium carbonate, 0.3mmol of pivalic acid, 0.3mmol of 4-methylphenylboronic acid, and 0.75mmol of toluene into the dry Schlenk tube, replace the gas atmosphere in the Schlenk tube from air to nitrogen, and replace the system three times in a nitrogen atmosphere of a standard atmospheric pressure to ensure that the system has a pure nitrogen atmosphere; under a nitrogen atmosphere, add 0.3mmol 3-bromo-4-iodotoluene and 2mL of toluene, heated to 120°C, stirred for 24h, cooled to room temperature; added 4mL of saturated ammonium chloride solution to quench the reaction, added 8mL of water, extracted with ethyl acetate, separated by column chromatography, and purified to obtain tolan Derivatives, the separation yield of this embodiment reaches 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com