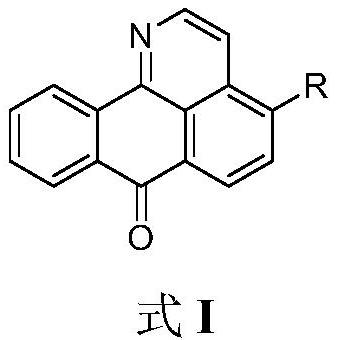
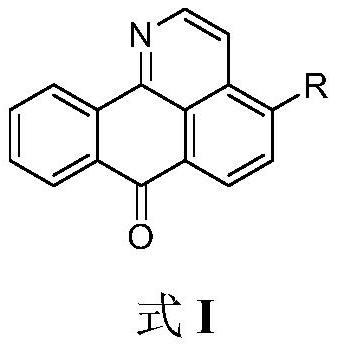
Isoaporphine oxide alkaloid derivative and application thereof
A technology of isoaporphine and derivatives, which is applied in the preparation of anticancer drugs, oxidized isoapofthene alkaloid derivatives and the field of preparation thereof, and can solve the problems of toxicity, side effect, solubility, stability and the like of natural products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: 4-[(2-morpholine) ethoxy]-1-azabenzanthrone (I 1 )Synthesis
[0031] The reaction formula is as follows:
[0032]
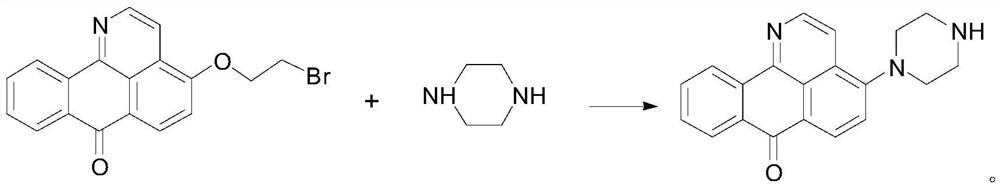
[0033] 4-Hydroxy-1-azabenzanthrone (1eq) was dissolved in 5mL of anhydrous DMF, and K 2 CO 3 (1.5eq) and 1,2-dibromoethane (1eq), stirred overnight at 80°C, stopped the reaction, added ethyl acetate and saturated brine to the reaction solution to extract and remove DMF, silica gel column chromatography (eluent was petroleum ether : ethyl acetate P / E=3:1) purification to obtain intermediate 4-(2-bromoethoxy)-1-azabenzanthrone, and then 4-(2-bromoethoxy)-1 -Azabenzanthrone (1eq) was added to the reaction flask, dissolved with 5mL anhydrous DMF, added K 2 CO 3 (1.5eq), CTAB and morpholine (1.2eq), stirred overnight at 60°C, stopped the reaction, extracted and removed DMF, and purified by silica gel column chromatography (eluent: petroleum ether:ethyl acetate P / E=5:1) to obtain Compound I 1 , yellow solid, yield 67%. Compound I 1 Iden...
Embodiment 2
[0036] Embodiment 2: Compound 4-[(2-isopropylamino)ethoxy]-1-azabenzanthrone (I 2 )Synthesis
[0037] The reaction formula is as follows:
[0038]
[0039] Reference compound I 1 The preparation method, replaces compound morpholine with isopropylamine, and other conditions are unchanged, obtains target compound I 2 , yellow solid, yield 58%. Compound I 2 Identified as 4-[(2-isopropylamino)ethoxy]-1-azabenzanthrone.
[0040] ESI-MS: 333.2.[M+H] + .
[0041] 1 H-NMR (300MHz, CDCl 3 , ppm), δ H8.86(d, J=9Hz, 1H), 8.77(d, J=6Hz, 1H), 8.48(d, J=9Hz, 1H), 8.28(d, J=9Hz, 1H), 7.98(d, J= 6Hz, 1H), 7.87(t, J=7.5Hz, 1H), 7.73(t, J=7.5Hz, 1H), 7.60(d, J=9Hz, 1H), 4.6(s, 1H), 3.96(t ,J=6Hz,2H),3.45(s,3H),1.24(d,J=6Hz,6H).
Embodiment 3
[0042] Embodiment 3: 4-[2-(2-methylpiperidine) ethoxy]-1-azabenzanthrone (I 3 )Synthesis
[0043] The reaction formula is as follows:
[0044]
[0045] Reference compound I 1 The preparation method, replaces compound morpholine with 2-methylpiperidine, other conditions are unchanged, obtains target compound I 3 , yellow solid, yield 80%. Compound I 3 Identified as 4-[2-(2-methylpiperidine)ethoxy]-1-azabenzanthrone.
[0046] ESI-MS: 373.2[M+H] + .
[0047] 1 H-NMR (300MHz, CDCl 3 , ppm), δ H 8.96(d, J=6Hz, 1H), 8.82(d, J=3Hz, 1H), 8.69(d, J=6Hz, 1H), 8.48(d, J=3H, 1H), 8.10(d, J= 3Hz, 1H), 7.84(t, J=4.5Hz, 1H), 7.69(t, J=4.5Hz, 1H), 7.23(d, J=3Hz, 1H), 4.43(t, J=3Hz, 2H) ,3.32(d,J=3Hz,1H),3.06(t,J=3Hz,2H),2.53(t,J=6Hz,J=9Hz,2H),1.72(t,J=6Hz,J=9Hz, 6H), 1.25(d, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com