Application of 6-aza-benzophenanthrene derivative to organic light-emitting element
A technology of organic light-emitting elements and organic light-emitting layers, which is applied in the direction of electrical components, organic chemistry, and electric solid-state devices, can solve the problems of reducing the luminous efficiency of organic light-emitting layers, reducing the current density of OLEDs, and shortening the service life of OLEDs, etc., to achieve good electrical conductivity properties, high electron affinity, and increased service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
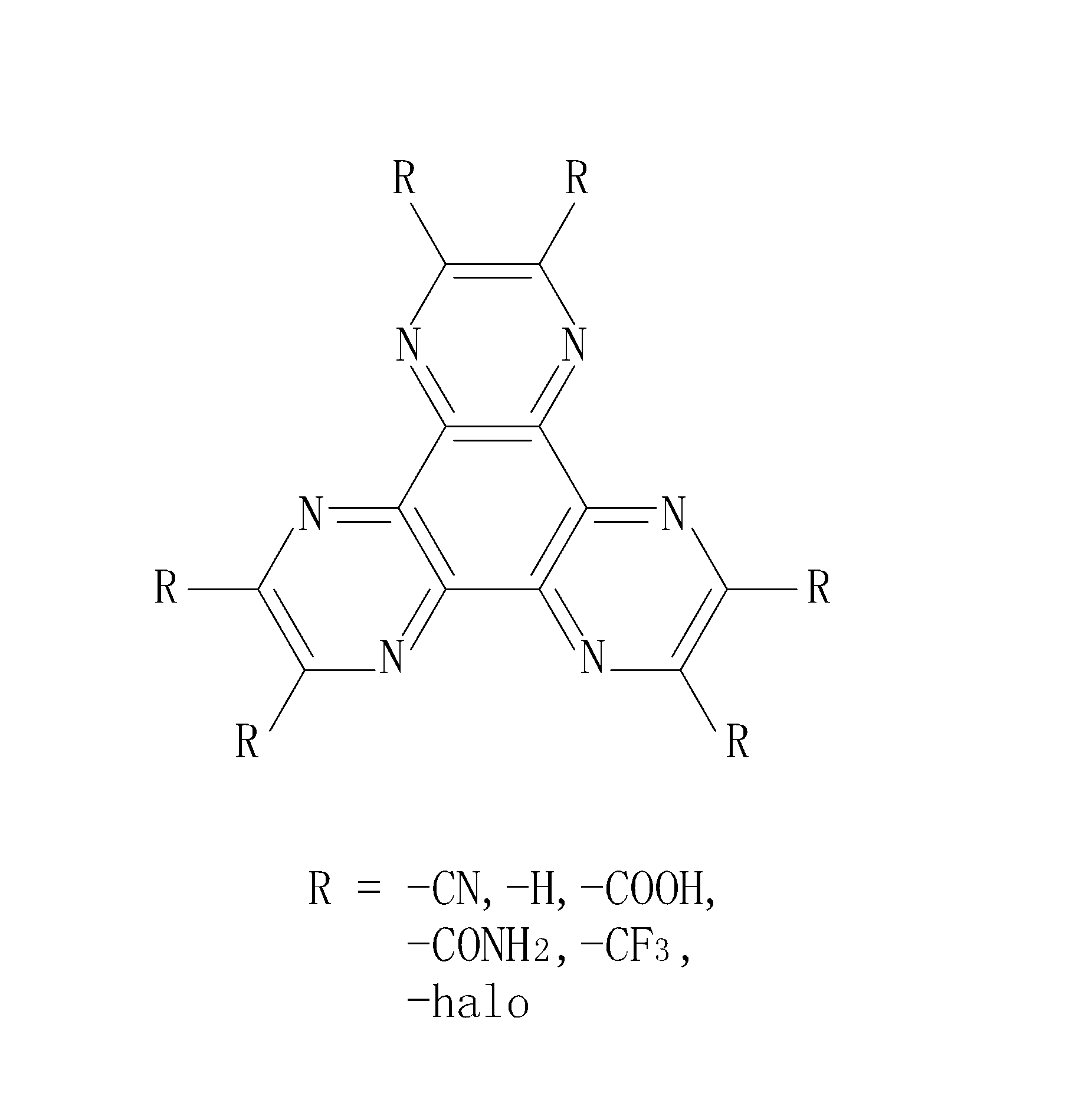
[0025] figure 1 It is the chemical formula of hexaazatriphenylene derivatives (HAT derivatives). See figure 1 As shown, the hexaazatriphenylene derivative contains four benzene rings, of which three peripheral benzene rings surround the central benzene ring, each peripheral benzene ring shares two carbon atoms with the central benzene ring, and two nitrogen atoms replace the two carbon atoms, and two non-shared carbon atoms are connected to two functional groups (functional group) R, wherein each functional group R is independently or simultaneously selected from nitrile (nitril, -CN), hydrogen (hydrogen, - H), ve carboxyl (carboxylic, -COOH), formamide (carboxamide, -CONH 2 ), trifluoromethyl (trifluoromethyl, -CF 3 ) and halogen (halogen, -halo). Compared with the existing organic semiconductor materials, the lowest unoccupied molecular orbital (LUMO) energy level value of hexaazatriphenylene derivatives is lower, so it has higher electron affinity and good conductivity,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com