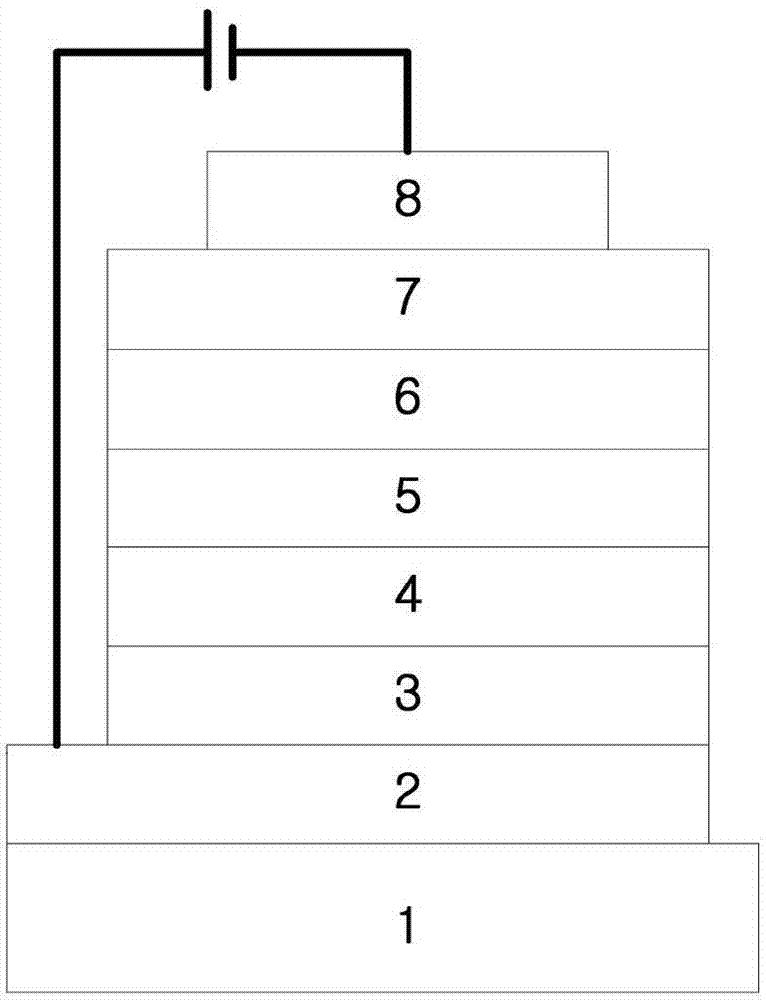
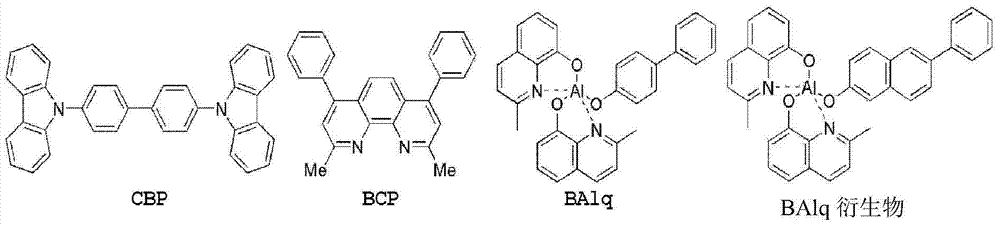
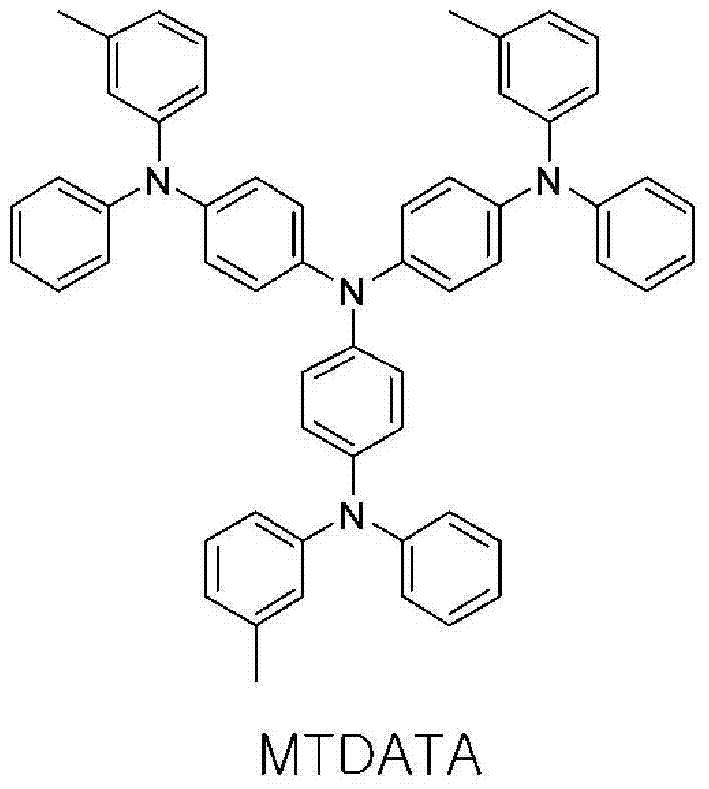
Novel compounds for organic electronic material and organic electronic device using the same
A technology of organic electronic materials and compounds, applied in the fields of organic chemistry, compounds of group 4/14 elements of the periodic table, compounds of group 5/15 elements of the periodic table, etc., can solve problems such as unsatisfactory luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0249] Preparation of Example 1 Compound (1)
[0250]
[0251] Preparation of compound (A)
[0252] Carbazole (20g, 119.6mmmol), methyl 2-iodobenzoate (26.4mL, 179.4mmol), K 2 CO 3 (21.5g, 155.5mmol), Cu (1.52g, 23.9mmol) and CuI (1.14g, 5.98mmol) were dissolved in dibutyl ether (500mL), and the solution was stirred at reflux for 48 hours under an argon atmosphere. After the reaction was completed, the reaction mixture was cooled to room temperature and extracted with water (800 mL). The organic layer was evaporated under reduced pressure. The residue was purified by column chromatography with hexane and ethyl acetate (4:1), and recrystallized from ethanol (300 mL) to obtain compound (A) (24.5 g, 68%).
[0253] Preparation of compound (B)
[0254] Compound (A) (15.0 g, 49.8 mmol) was dissolved in diethyl ether (100 mL), and the solution was frozen to -78°C. Methyl lithium (1.6M in diethyl ether, 78 mL, 124.4 mmol) was added thereto, and the mixture was stirred for 1 h...
preparation Embodiment 2
[0261] Preparation of Preparation Example 2 Compound (320)
[0262]
[0263] Preparation of compound (E)
[0264] Compound (C) (7 g, 24.7 mmol) was dissolved in dichloromethane (100 mL), and N-bromosuccinimide (10.5 g, 59.2 mmol) was added thereto at 0°C. Maintaining this temperature, the solution was stirred for 5 hours. Distilled water (150 mL) was added to terminate the reaction, the mixture was extracted with dichloromethane (100 mL), and the extract was evaporated under reduced pressure. Compound (E) (7.4 g, 68%) was obtained by recrystallization from ethanol / acetone (1:2 v / v).
[0265] Preparation of compound (320)
[0266] Compound (E) (5g, 13.8mmol), phenylboronic acid (3.8g, 30.36mmol) and Pd(PPh 3 ) 4 (1.6 g, 1.04 mmol) was dissolved in toluene (100 mL) and ethanol (50 mL), and 2M aqueous sodium carbonate solution (50 mL) was added thereto. After stirring under reflux at 120°C for 4 hours, the reaction mixture was cooled to 25°C, and distilled water (200 mL)...
preparation Embodiment 3
[0267] Preparation of Preparation Example 3 Compound (462)
[0268]
[0269] Preparation of compound (F)
[0270] Carbazole (20g, 119.6mmmol), methyl 5-bromo-2-iodobenzoate (26.4mL, 179.4mmol), K 2 CO 3 (21.5g, 155.5mmol), Cu (1.52g, 23.9mmol) and CuI (1.14g, 5.98mmol) were dissolved in dibutyl ether (500mL), and the solution was stirred at reflux for 48 hours under an argon atmosphere. After the reaction was completed, the reaction mixture was cooled to room temperature and extracted with water (800 mL). The organic layer was evaporated under reduced pressure. The residue was purified by column chromatography with hexane and ethyl acetate (4:1), and recrystallized from ethanol (300 mL) to obtain compound (F) (24.5 g, 58%).
[0271] Preparation of compound (G)
[0272] Compound (F) (15.0 g, 49.8 mmol) was dissolved in diethyl ether (100 mL), and the solution was frozen to -78°C. Methyl lithium (1.6M in diethyl ether, 78 mL, 124.4 mmol) was added thereto, and the compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com