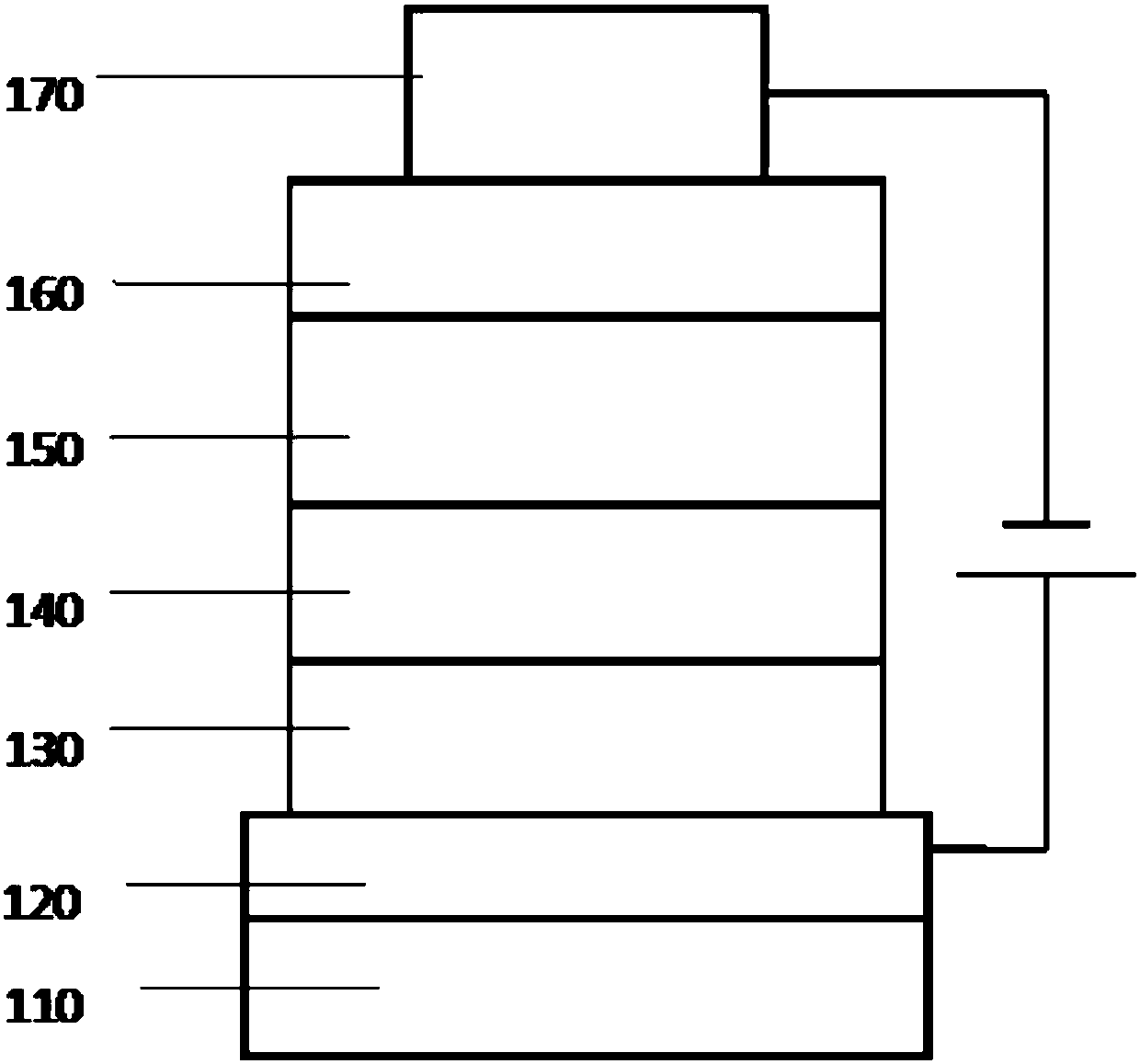
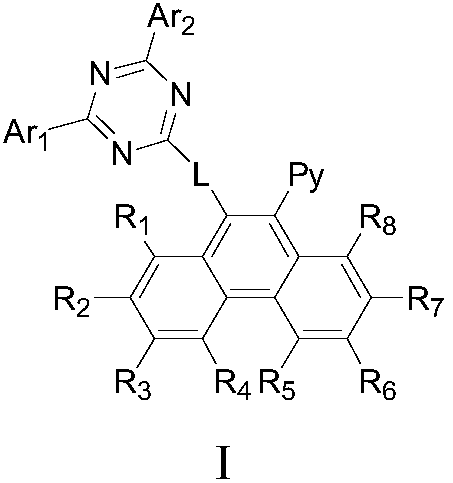
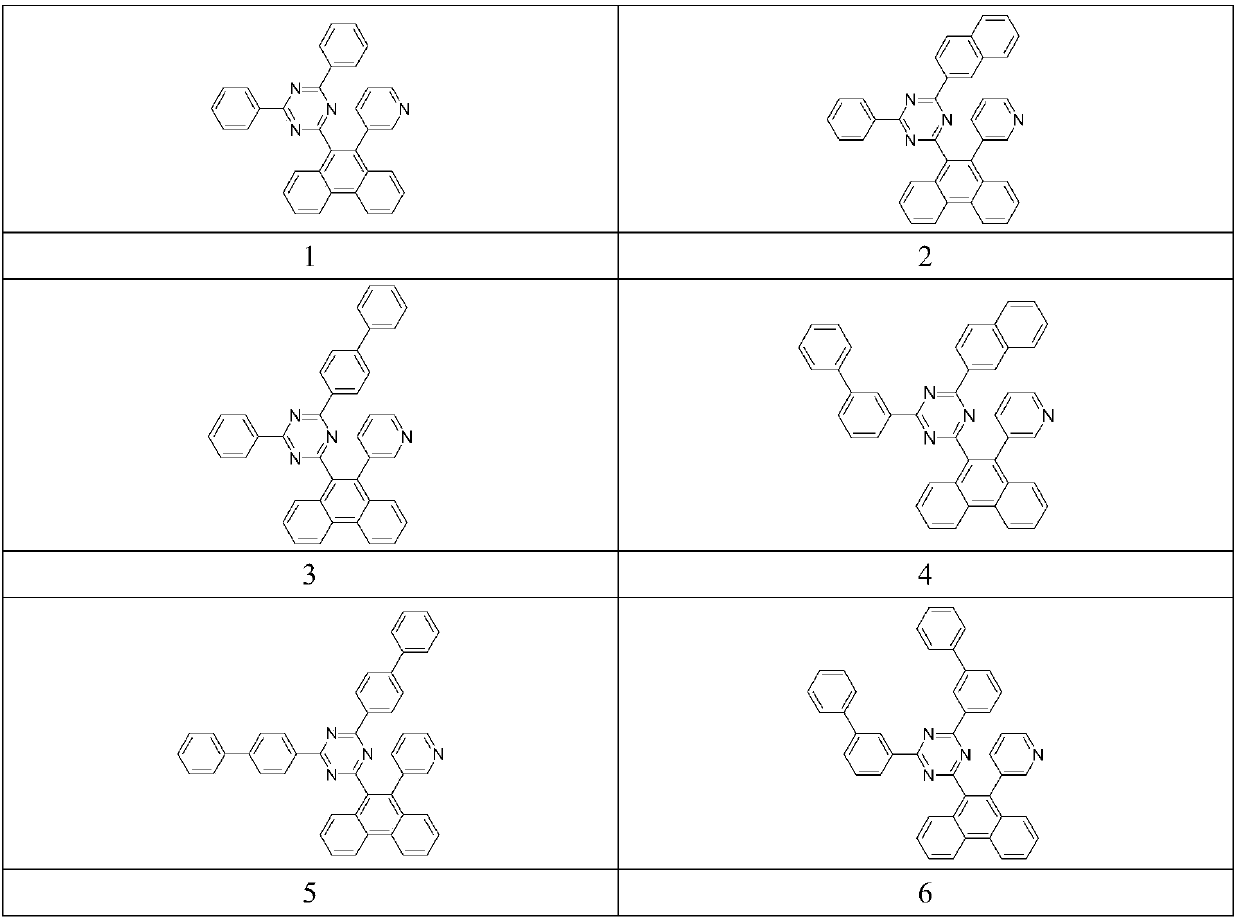
Phenanthrene derivatives, applications thereof, and organic electroluminescent device
A derivative and phenanthrene-based technology, applied in the field of organic electroluminescent devices, can solve problems such as low electron mobility, low electron mobility, and affecting device life and efficiency, and achieve high luminous purity, high luminous efficiency, and good heat dissipation. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthetic route of compound 1
[0046]
[0047] The synthetic method of intermediate 1-1
[0048] In a flask, add 9,10-dibromophenanthrene (15g, 45mmol), pinacol 3-pyridine borate (11g, 54mmol), potassium carbonate (7.5g, 54mmol), tetrahydrofuran (150mL), water (100mL) , tetrakistriphenylphosphine palladium (1g), heated to reflux for 12 hours under nitrogen protection, cooled, extracted with dichloromethane, dried, concentrated, the crude product was purified by column chromatography to obtain 8.7g, yield 58%.
[0049] The synthetic method of intermediate 1-2
[0050] In a flask, add Intermediate 1-1 (8 g, 24 mmol), pinacol diboronate (9.1 g, 36 mmol), potassium acetate (3.5 g, 36 mmol), dioxane (120 mL), Pd(dppf) C l2 (0.5g), heated to reflux under nitrogen protection for 12 hours, cooled, removed the solvent, added water, extracted with dichloromethane, dried, concentrated, and the crude product was purified by column chromatography to obtain 5.9g, yield 65%.
...
Embodiment 2
[0054] Synthetic route of compound 5
[0055]
[0056] The synthesis method is the same as that of intermediate 1-1, the raw materials used are intermediate 1-2 and 2-chloro-3,5-bis(4-phenylbenzene)-triazine, and the yield is 52%.
Embodiment 3
[0058] Synthetic route of compound 7
[0059]
[0060] The synthetic method of intermediate 7-1
[0061] The synthesis method is the same as that of intermediate 1-1, the raw materials used are 9,10-dibromophenanthrene and 2-phenyl-5-pinacol borate pyridine, and the yield is 47%.
[0062] The synthetic method of intermediate 7-2
[0063] The synthesis method is the same as that of intermediate 1-2, the raw material used is intermediate 7-1, and the yield is 55%.
[0064] The synthetic method of compound 7
[0065] The synthesis method is the same as that of intermediate 1-1, the raw materials used are intermediate 7-2 and 2-chloro-3,5-diphenyltriazine, and the yield is 46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com