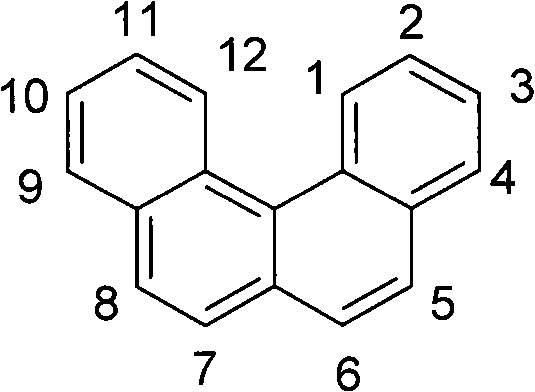
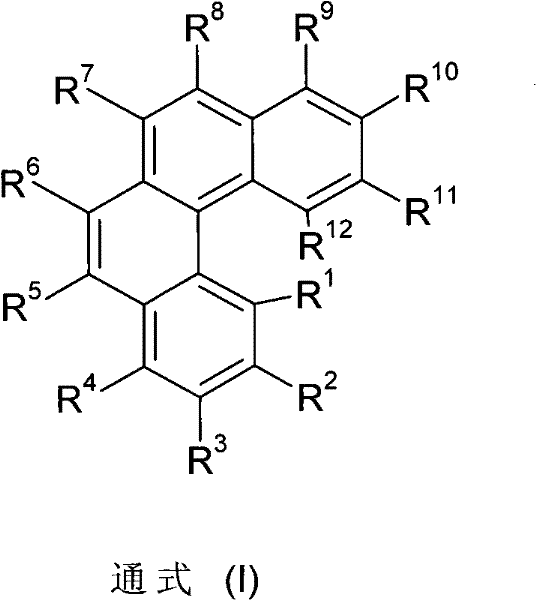
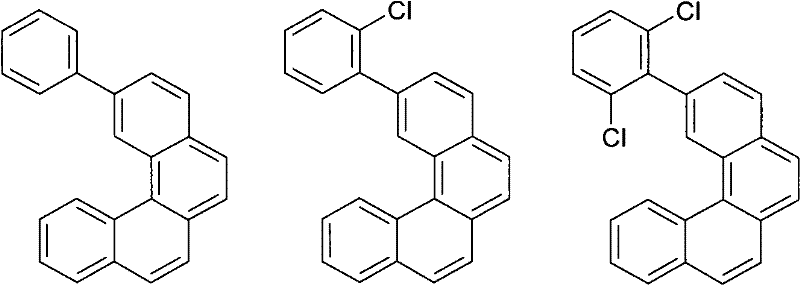
Materials for Organic Electroluminescent Devices
A compound, general formula technology, applied in the field of organic semiconductors, can solve the problems of high technical complexity and instability of OLED manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] Embodiment 1: Synthesis of 2,11-bis(naphthalene-1-yl)benzo[c]phenanthrene
[0159] a) Synthesis of two p-bromobenzylidene acetone
[0160]
[0161] 296g (1600mmol) p-bromobenzaldehyde was added dropwise to the solution of 52.8g (800mmol) potassium hydroxide (85%) and 58.9ml (800mmol) acetone in 1.6L water and 2L ethanol, and the mixture was Stir overnight at room temperature. The precipitated solid was filtered off with suction, washed with 3 L of water and dried under vacuum. Yield: 284 g (826 mmol), 91%.
[0162] b) Synthesis of 1,5-bis(p-bromobenzyl)pentan-3-one
[0163]
[0164] 217g (555mmol) of di-p-bromobenzylideneacetone was suspended in 20ml of glacial acetic acid in a solution of 1L of ethyl acetate, 14g of Pd / C (5%) was added, and the mixture was placed in a 2.8L autoclave at 4 Bar H 2 Stir under pressure. When the hydrogen absorption was complete (about 30 minutes), the mixture was 2 Stir under pressure for an additional 2 hours. Filter to remo...
Embodiment 2
[0178] Example 2: Synthesis of 9-(phenyl)-10-(benzo[c]phenanthrene-5-yl)anthracene
[0179] a) Synthesis of benzo[c]phenanthrene-5-boronic acid
[0180]
[0181] 52ml (130mmol) of n-butyllithium (2.5M in n-hexane) was added dropwise to a suspension of 30.7g (100mmol) of 5-bromobenzo[c]phenanthrene in 1000ml of THF at -78°C under vigorous stirring solution, and the mixture was stirred for an additional 2 hours. 16.7ml (150mmol) trimethyl borate was added to the red solution in one portion under vigorous stirring, the mixture was stirred at -78°C for another 30 minutes, then warmed to room temperature within 3 hours, added 300 ml of water, and the mixture was stirred for 30 minutes. The organic phase was separated and evaporated to dryness under vacuum. The solid was dissolved in 100 ml of n-hexane, filtered with suction, washed once with 100 ml of hexane and dried under vacuum. Yield: 24.8 g (91 mmol), 91 %, about 90 % pure (NMR) boronic acid with varying amounts of bori...
Embodiment 3
[0185] Example 3: Synthesis of 9-(naphthalene-2-yl)-10-(benzo[c]phenanthrene-5-yl)anthracene
[0186]
[0187] 913 mg (3 mmol) tri-o-tolylphosphine, and then 112 mg (0.5 mmol) palladium(II) acetate were added to vigorously stirred 19.2 g (50 mmol) 9-bromo-10-(2-naphthyl)anthracene, 14.9 g ( 55mmol) benzo [c] phenanthrene-4-boronic acid and 25.5g (120mmol) tripotassium phosphate in 300ml toluene, 100ml di a suspension in a mixture of alkanes and 400 ml of water, and the mixture was then heated at reflux for 16 hours. After the mixture had cooled, the precipitated solids were filtered off with suction, washed three times with 50 ml of toluene, three times with 50 ml of ethanol:water (1:1, v:v), and three times with 100 ml of ethanol. / g) was recrystallized three times. Yield: 15.3 g (29 mmol), 58.8%, purity 99.9% (HPLC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com