Patents
Literature
37results about "Benzathrone dyes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
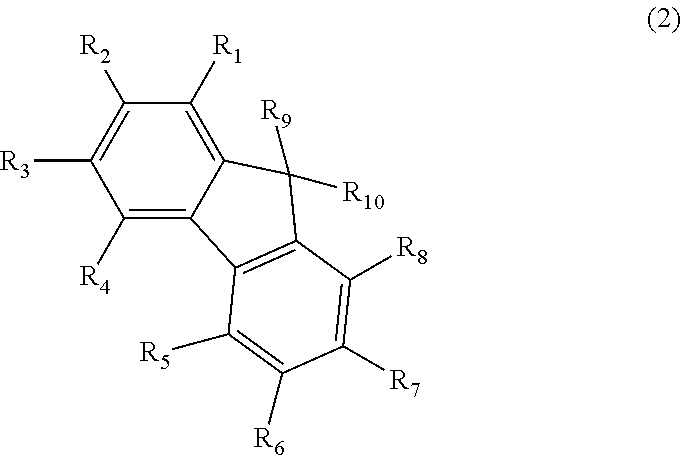
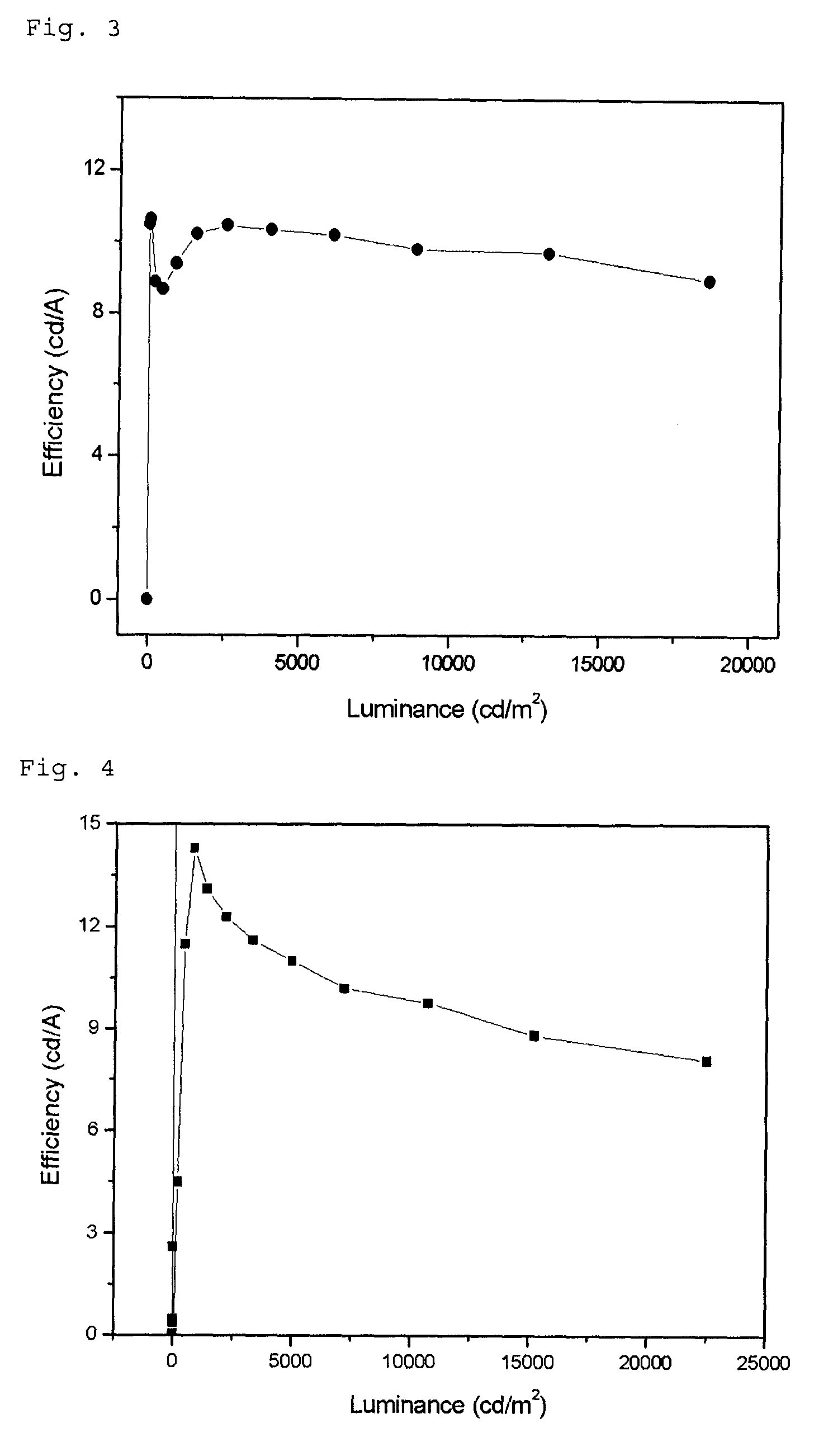
Benzanthracene derivative and electroluminescence device using the same
ActiveUS20070273272A1Improve efficiencyGood chromaSilicon organic compoundsDischarge tube luminescnet screensOrganic filmOrganic electroluminescence
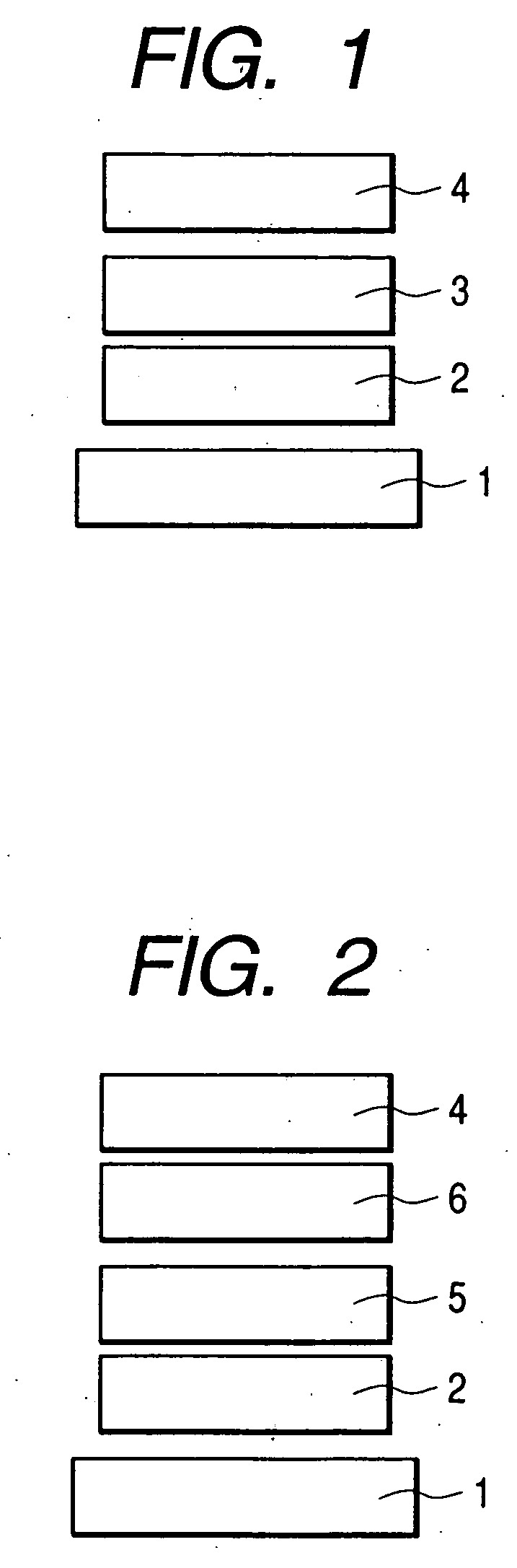
A benzanthracene derivative having hydrogen atom at the 12-position, and an organic electroluminescence device having an organic thin film layer, which has one layer or a plurality of layers including at least a light emitting layer, is disposed between a cathode and an anode and contains the benzanthracene derivative in at least one layer in the organic thin film layer singly or as a component of a mixture. The electroluminescence device provides a great efficiency of light emission, has a long life and exhibits an excellent chromaticity.
Owner:IDEMITSU KOSAN CO LTD
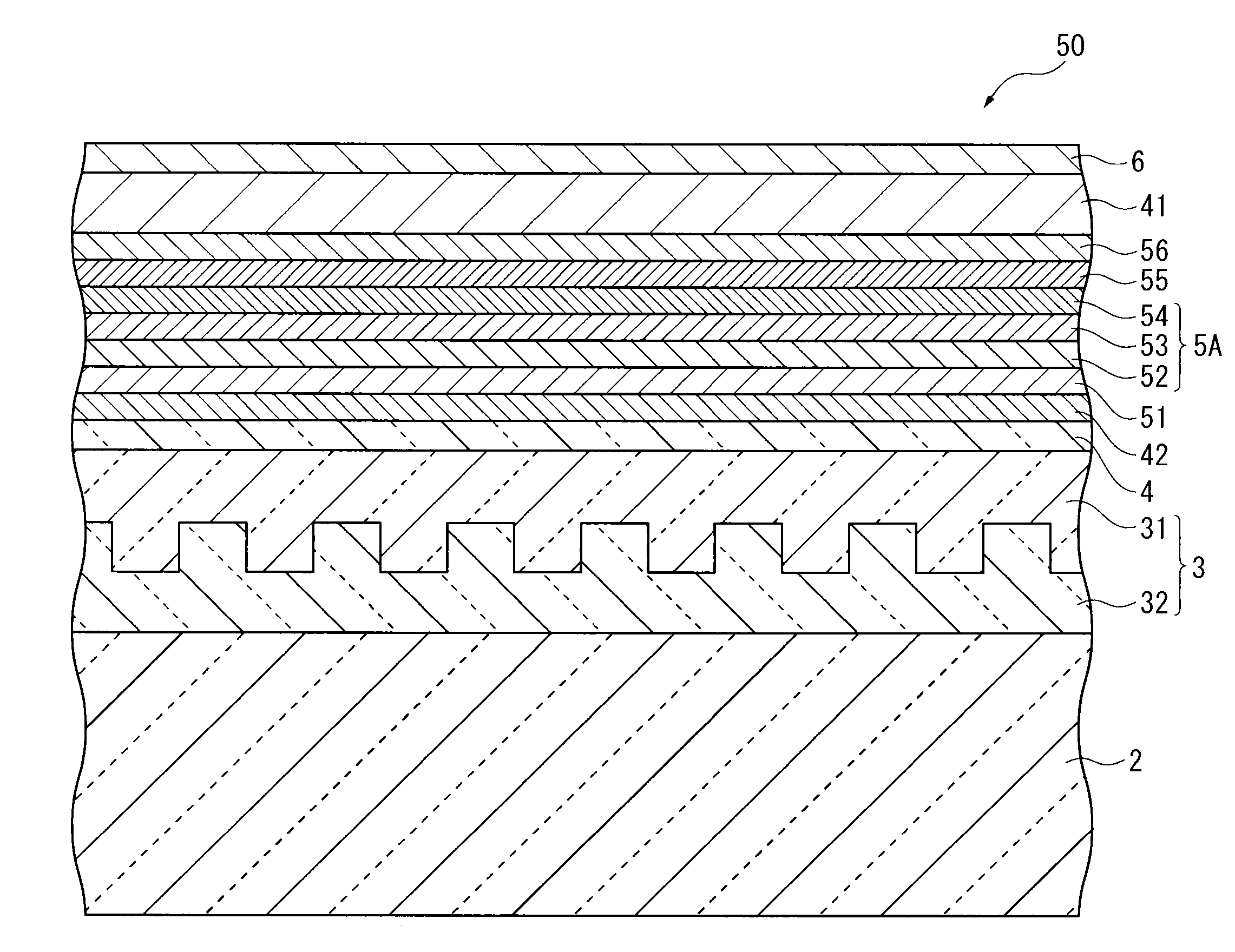
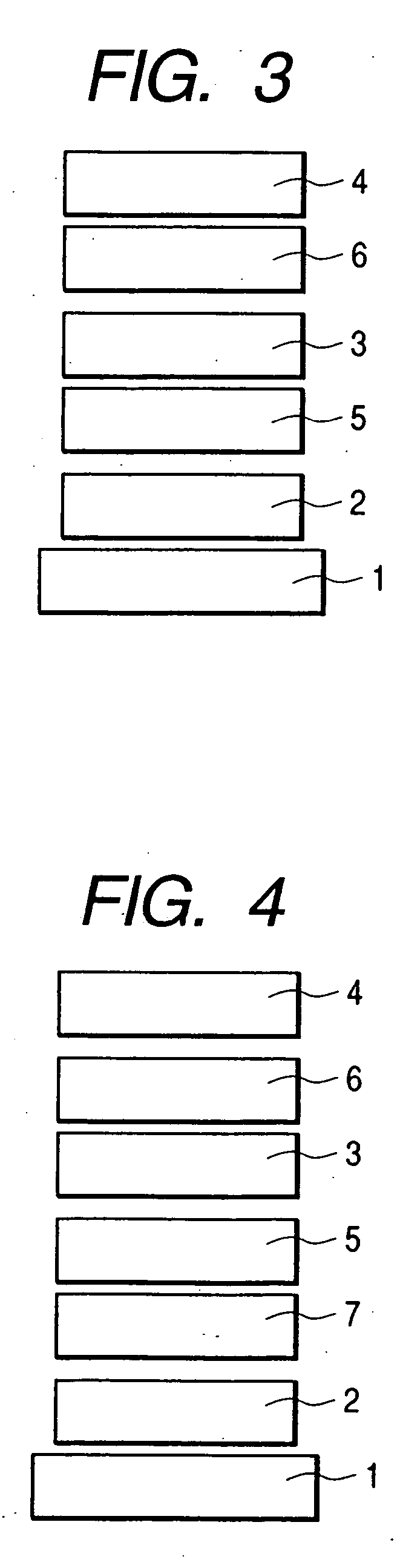
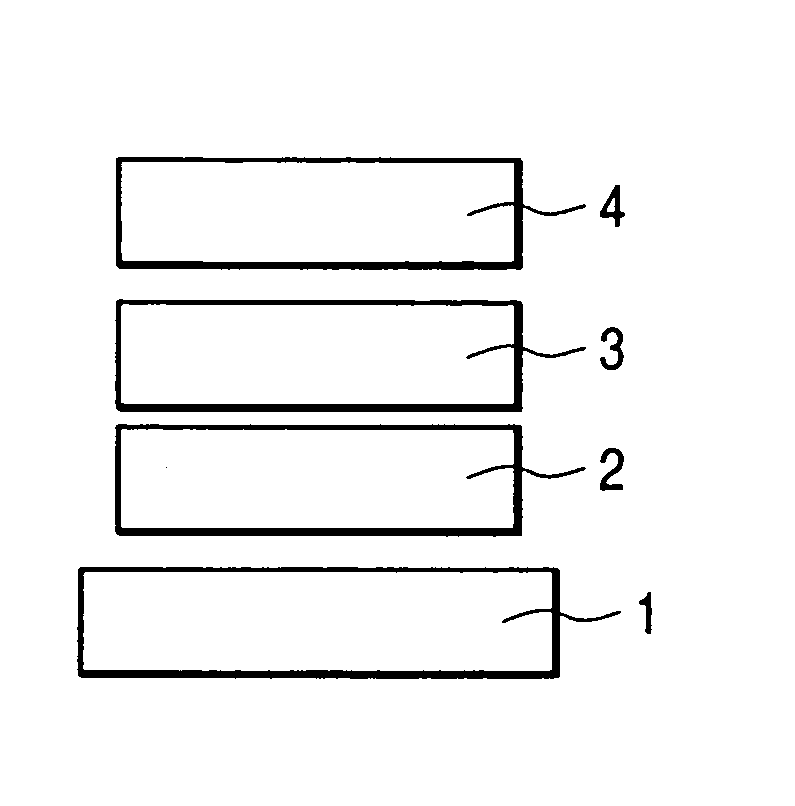
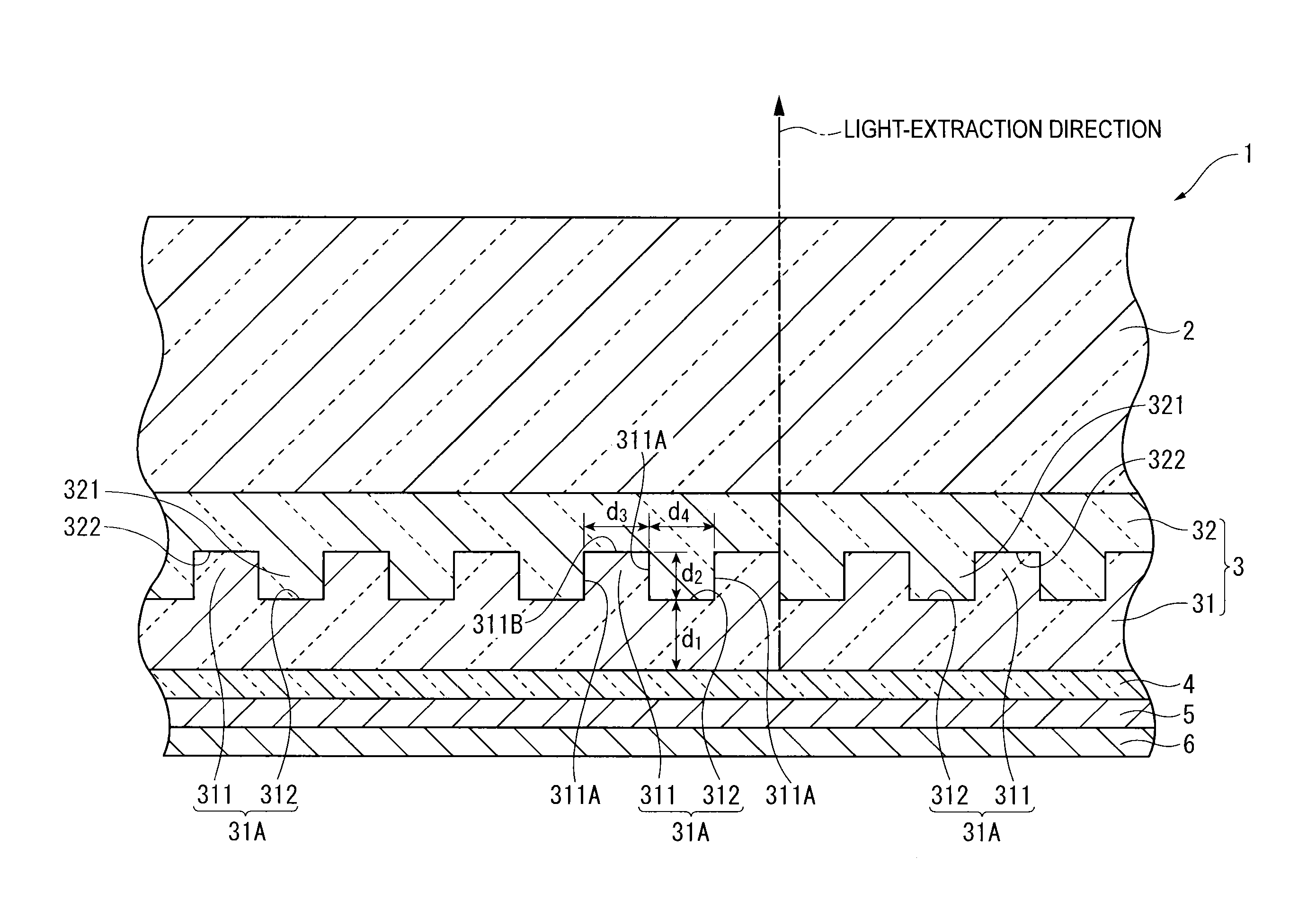
Organic electroluminescent element and lighting device
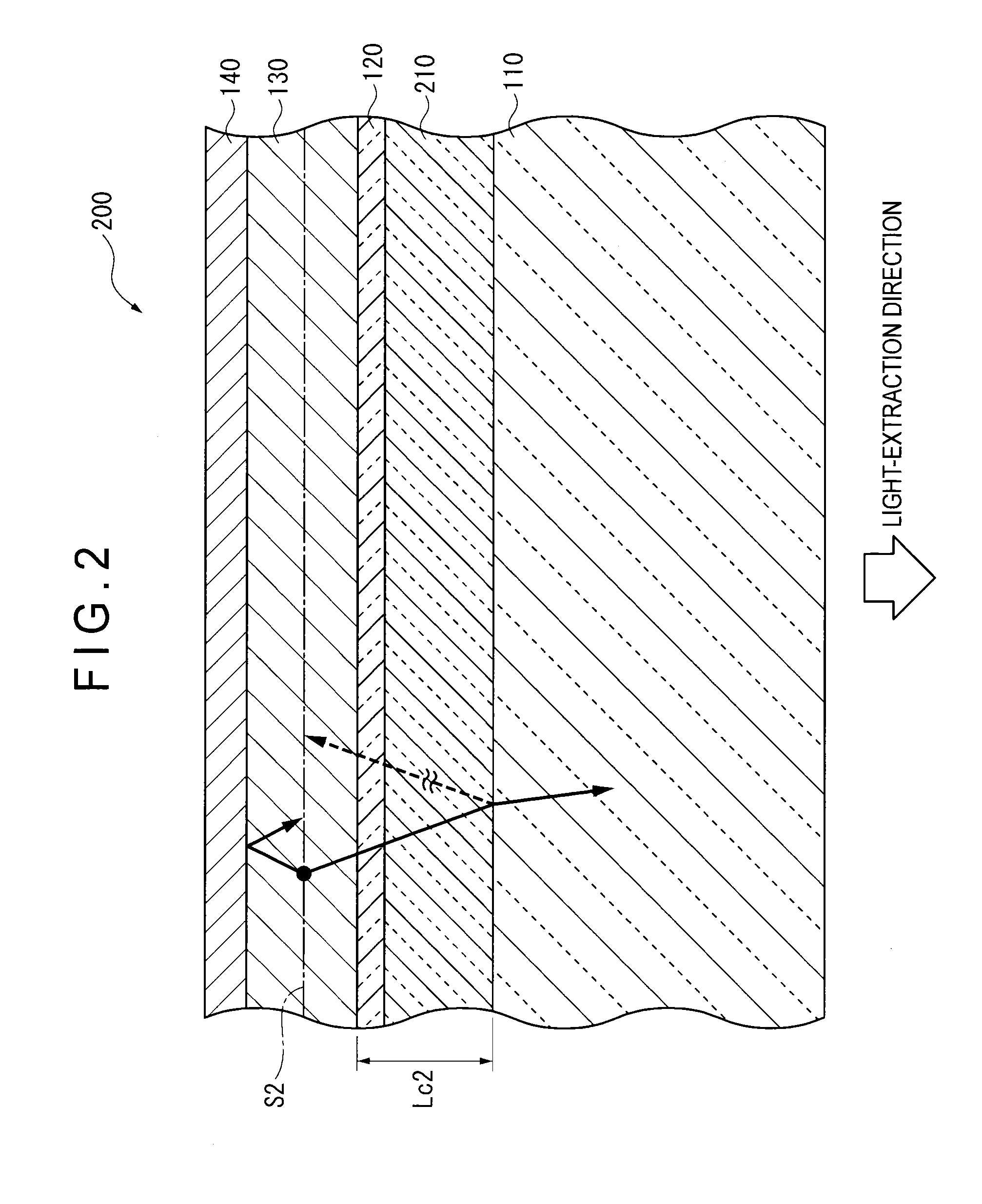
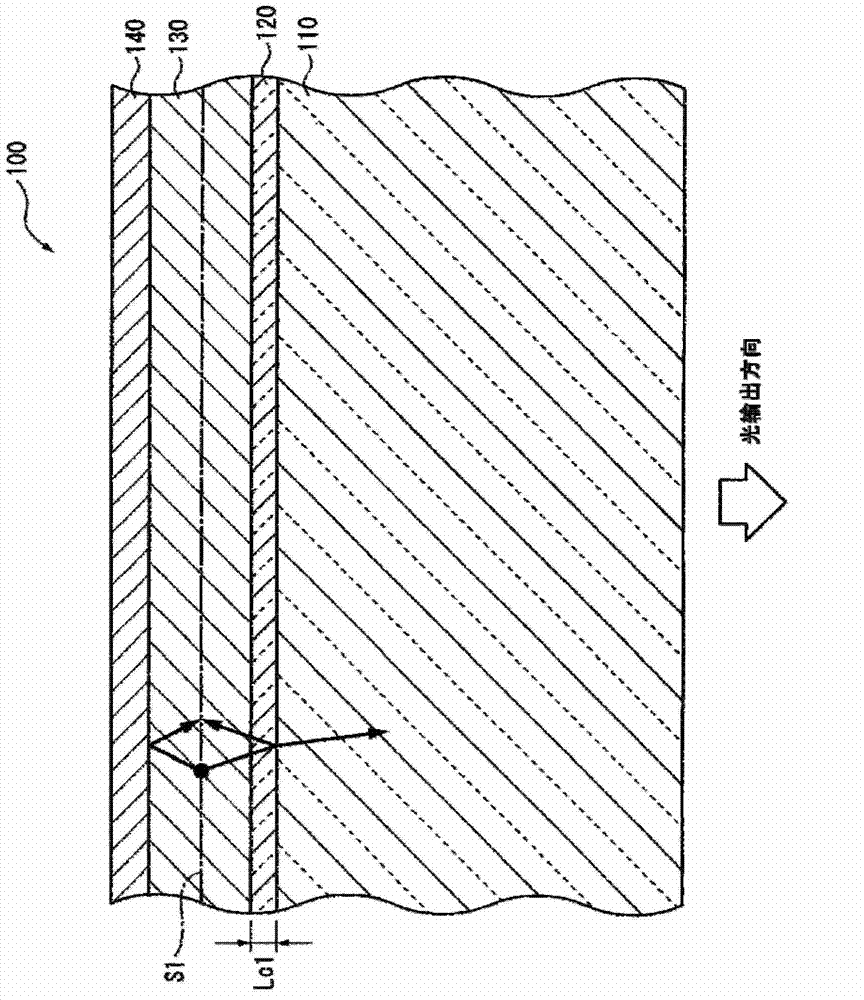
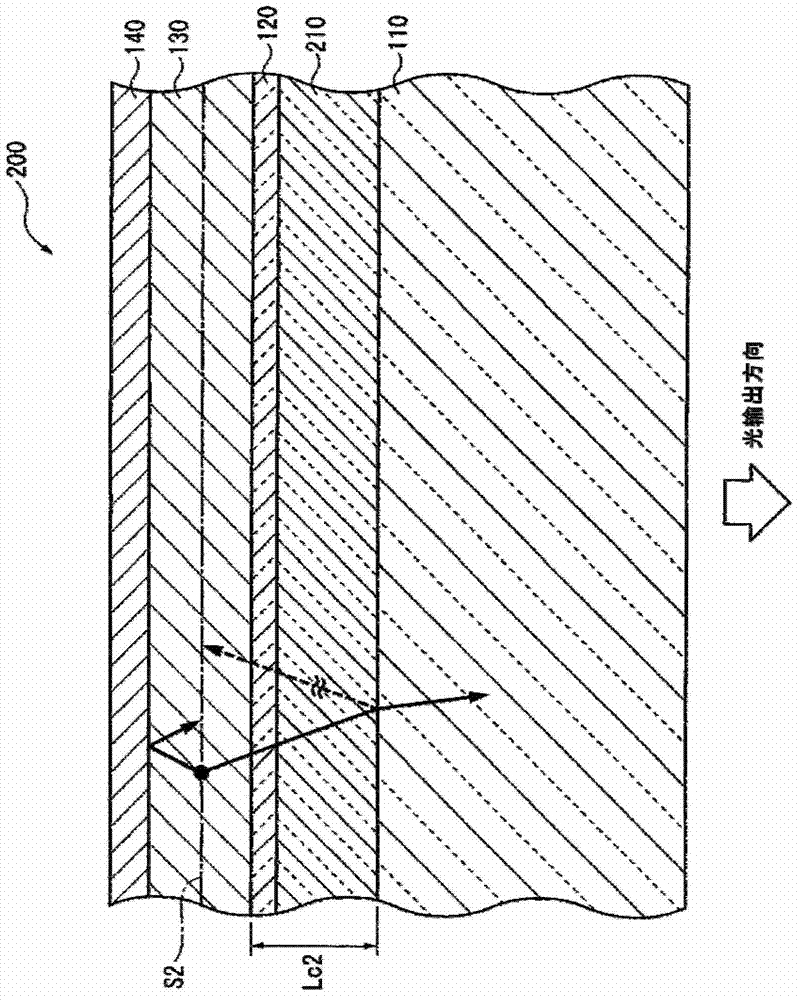
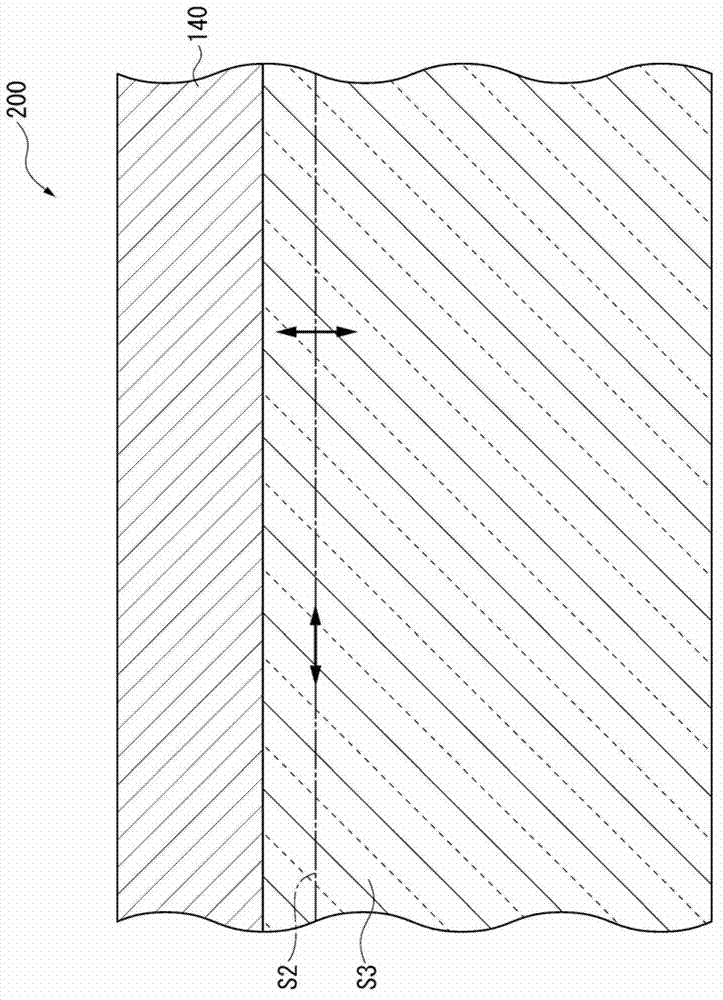
ActiveUS20120286258A1Improve efficiencyIncrease brightnessElectroluminescent light sourcesSolid-state devicesRefractive indexOrganic electroluminescence
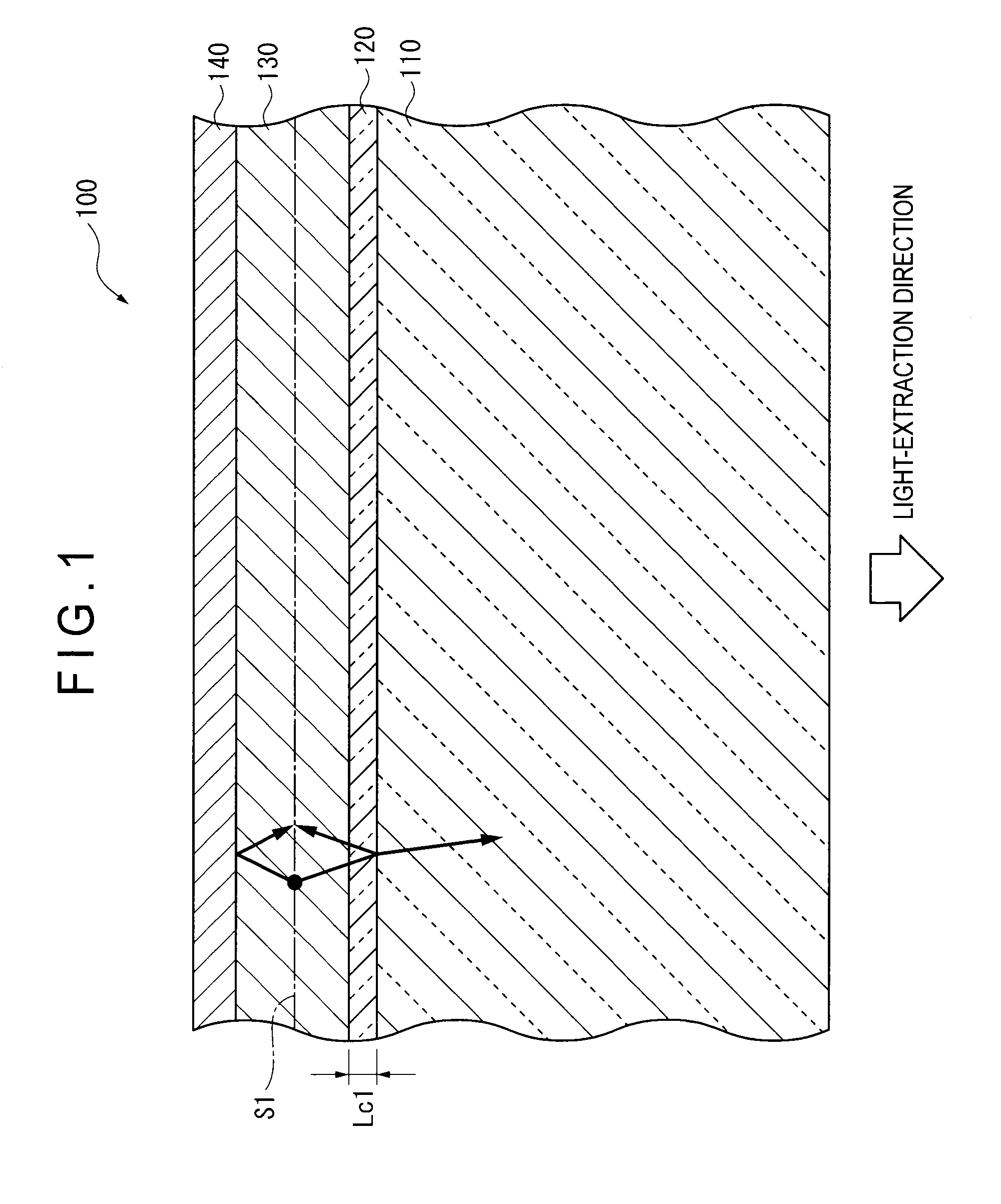
An organic EL device (1) comprises: a light-transmissive substrate (2); a transparent electrode (4); an organic emitting layer (5); and an opposing electrode (6). A light-extraction layer (3) provided by layering a high refractive index layer (31) adjoining the transparent electrode (4) and a low refractive index layer (32) adjoining the light-transmissive substrate (2) includes recess-protrusion units (31A) each provided by a protrusion (311) and a recess (312) at an interface between the high refractive index layer (31) and the low refractive index layer (32). In at least one of the recess-protrusion units (31A), a distance d1 from the interface between the high refractive index layer (31) and the low refractive index layer (32) to an interface between the transparent electrode (4) and the high refractive index layer (31) is equal to or more than an optical coherence length and a height d2 of the protrusion (311), a width d3 of the protrusion (311) and a gap d4 between the protrusion (311) and another protrusion (311) adjoining the protrusion across the recess (312) is 1 μm or more.
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescent element and lighting device
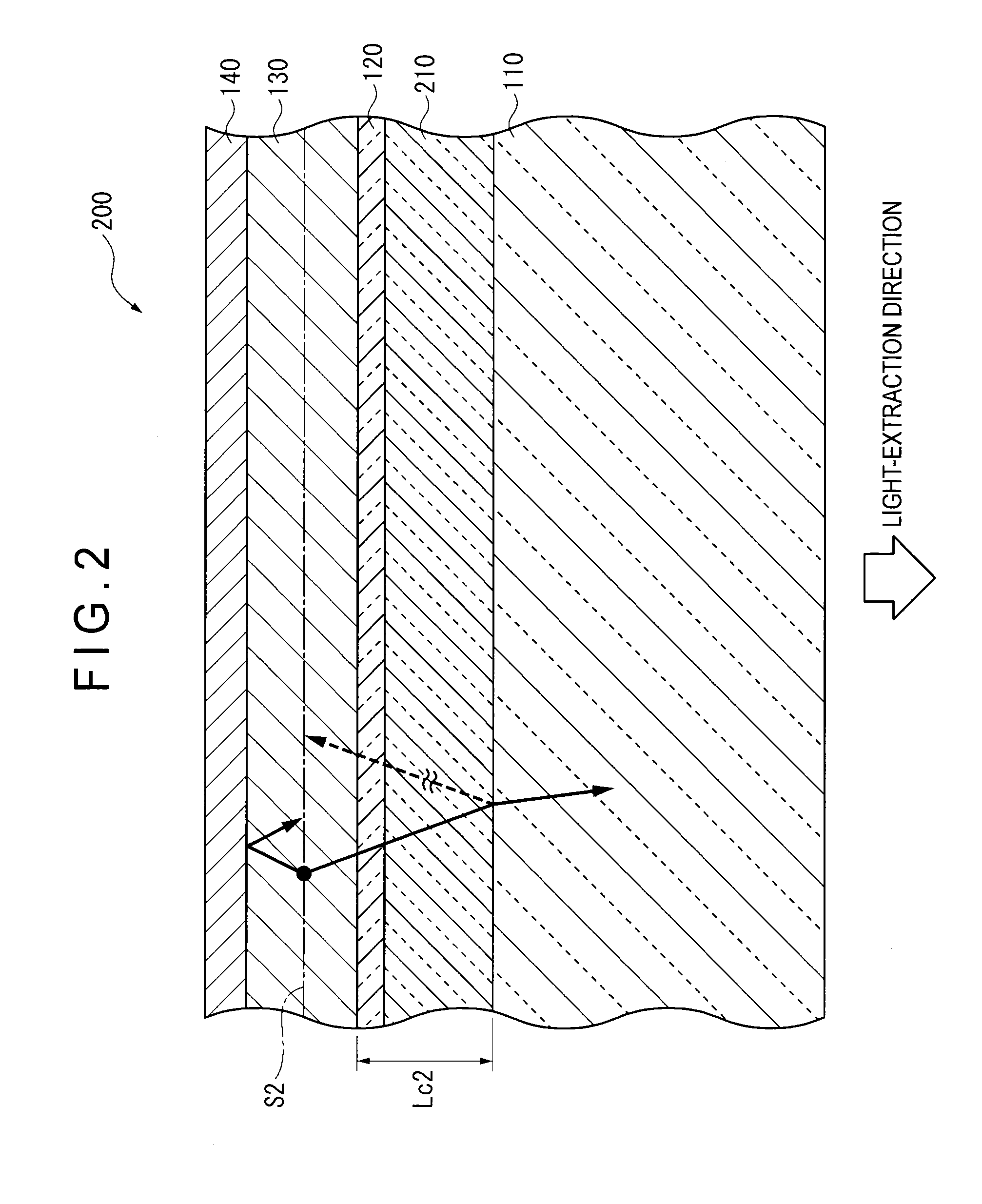
ActiveCN102742352AElectroluminescent light sourcesSolid-state devicesRefractive indexOrganic electroluminescence
Disclosed is an organic EL element (1)-which is provided with a counter electrode (6), an organic light-emitting layer (5), and a transparent electrode (4) on a translucent substrate (2)-wherein: a light extraction layer (3), of which a high refractive index layer (31) adjacent to the transparent electrode (4) and a low refractive index layer (32) adjacent to the translucent substrate (2) are stacked, has a plurality of irregular-section units (31A) configured from convex sections (311) and concave sections (312) at the interface of the high refractive index layer (31) and the low refractive index layer (32); in at least one of the plurality of irregular-section units (31A), the distance (d1) from the interface between the high refractive index layer (31) and the low refractive index layer (32) to the interface between the transparent electrode (4) and the high refractive index layer (31) is at least the optical coherence length; and the height (d2) of the convex sections (311), the width (d3) of the convex sections (311), and the spacing (d4) between each convex sections (311) and the adjacent convex sections (311) with concave sections (312) therebetween is at least 1 [mu]m.
Owner:IDEMITSU KOSAN CO LTD
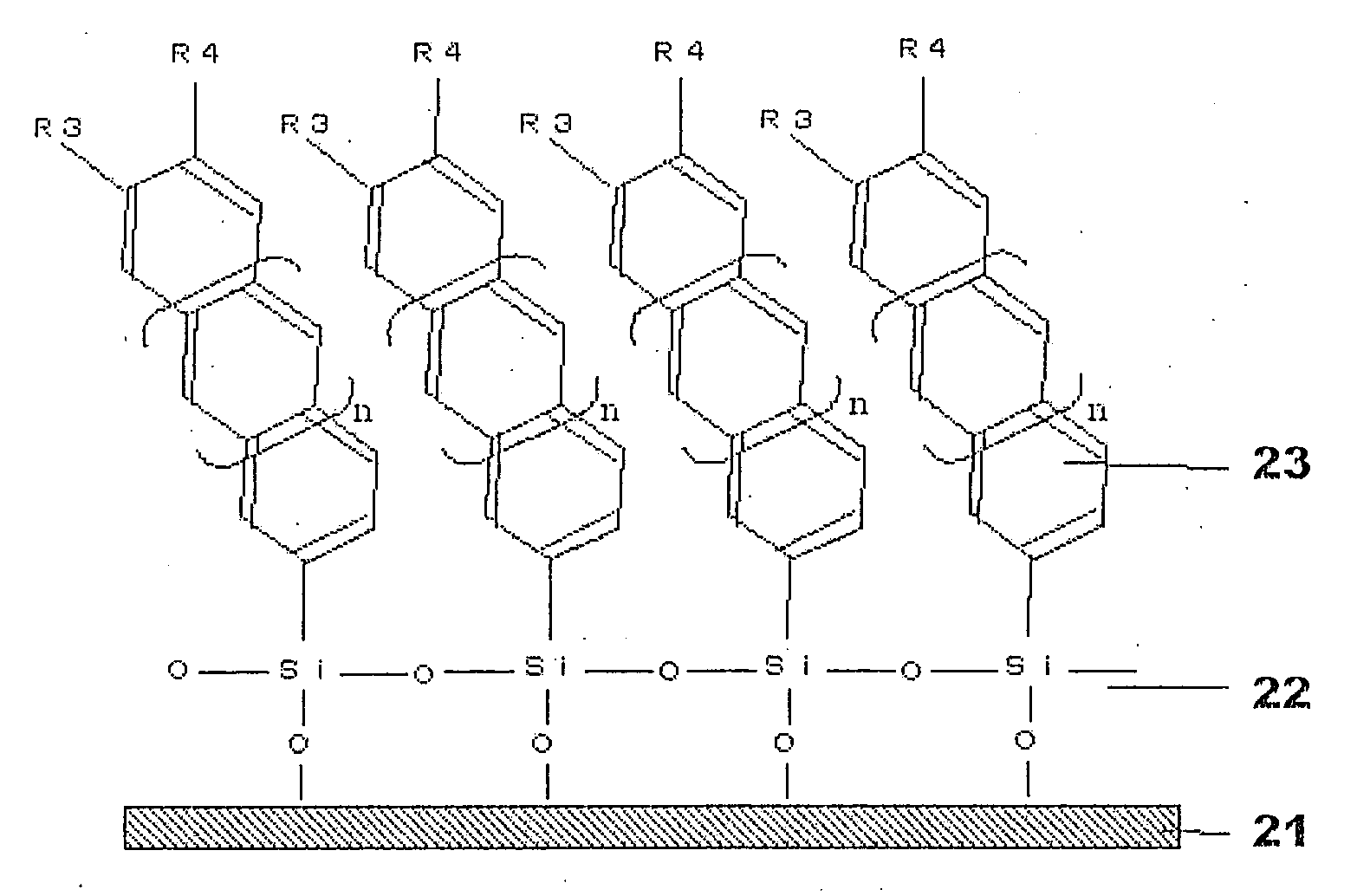
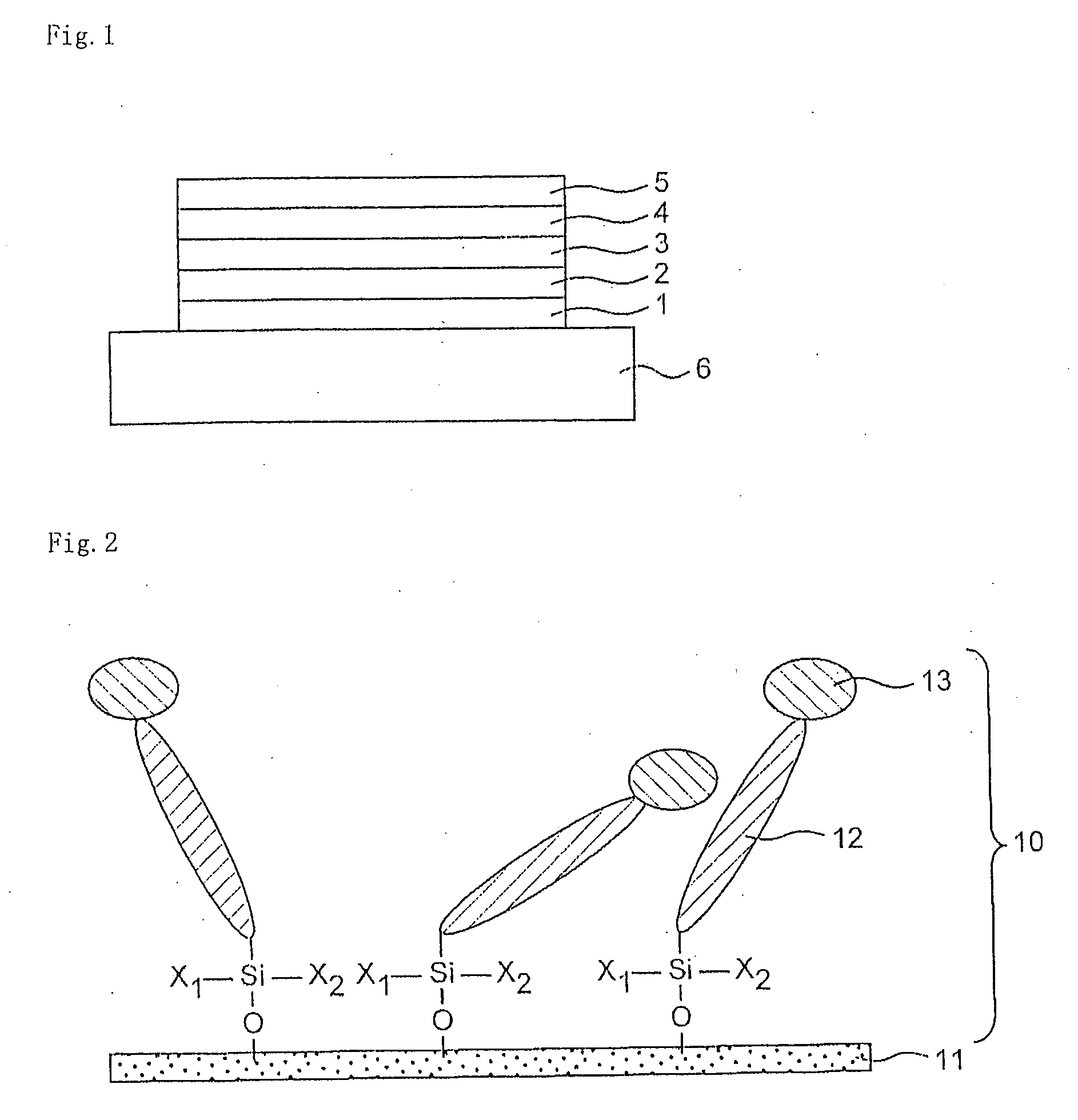
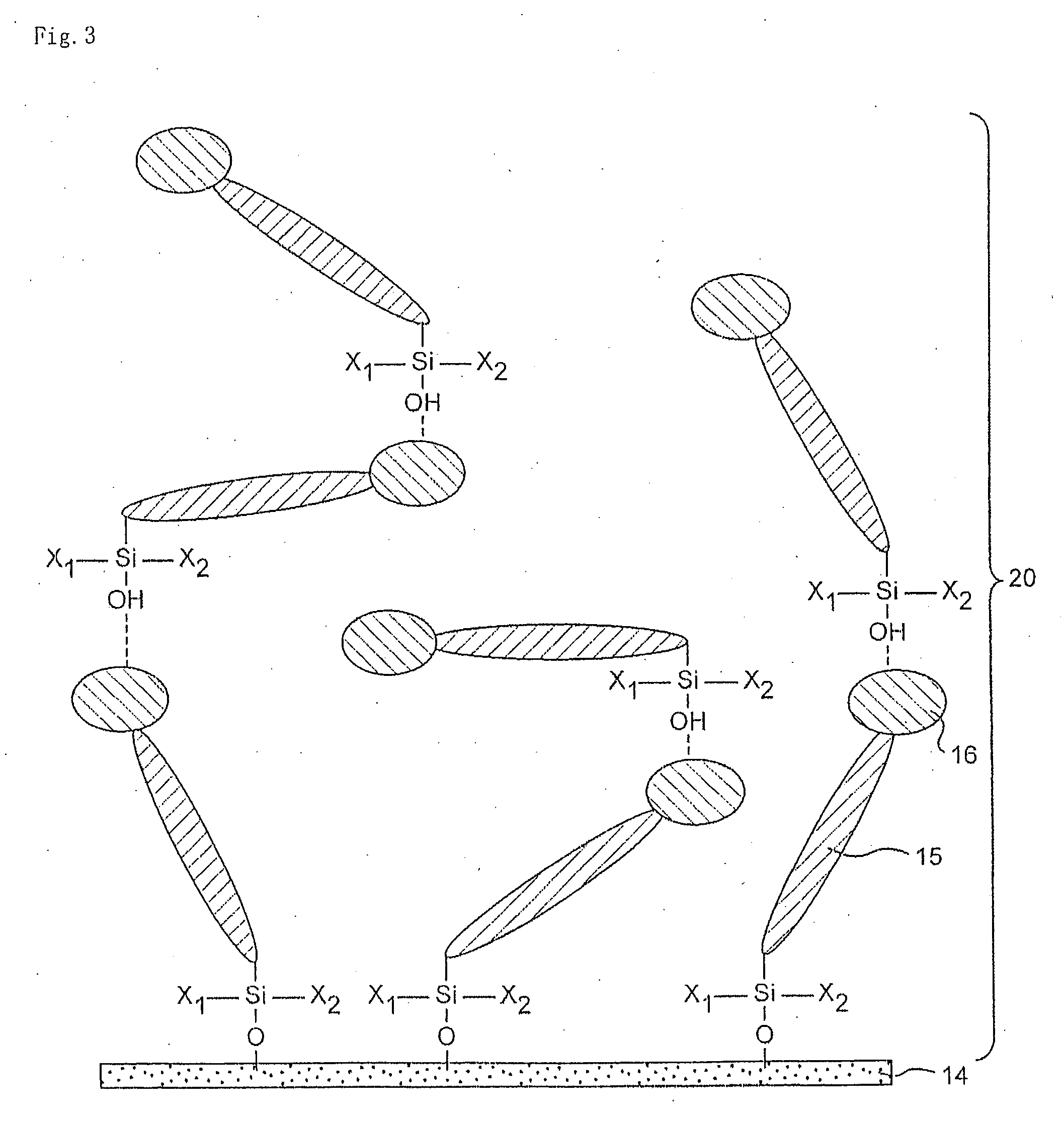
Organosilanes, Process For Production of the Same, and Use Thereof
InactiveUS20080207864A1Improve stabilityHigh stability and durabilitySilicon organic compoundsBenzathrone dyesSilane compoundsHalogen
An organic silane compound represented by the general formula (a); (T)k-SiX1X2X3 (a) wherein T is an organic group derived from a fused polycyclic hydrocarbon compound of a fusion number of 2 to 10 composed of a 5-membered and / or 6-membered monocyclic hydrocarbon; k is an integer of 1 to 10; at least one group of X1 to X3 is a group which gives a hydroxy group by hydrolysis, or a halogen atom, and other groups are a group which does not react with an adjacent molecule.
Owner:SHARP KK
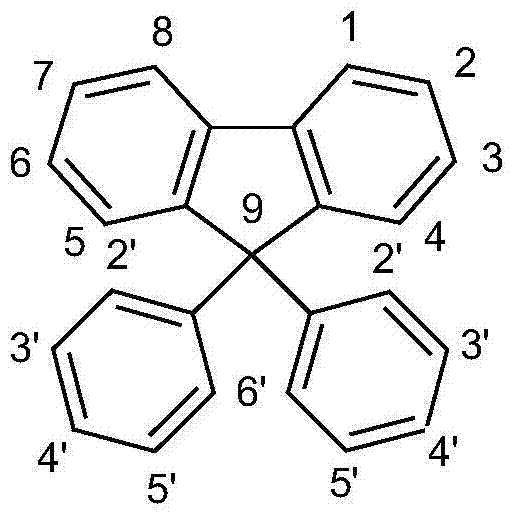
Spiro compound and organic luminescence device using the same
InactiveUS20060134425A1Improve efficiencyIncrease brightnessMethine/polymethine dyesSolid-state devicesArylHydrogen atom
Provided are a novel spiro compound, and an organic luminescence device using the spiro compound and having an optical output with an extremely high efficiency and a high luminance, and an extremely high durability. The Spiro compound is represented by the following general formula [I]: (wherein R1, R2, R3, and R4 represent a hydrogen atom, a substituted or unsubstituted alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted heterocyclic group, a substituted amino group, a cyano group, or a halogen atom, and R1, R2, R3, and R4 may be identical or different from each other; and Ar1 and Ar2 represent a substituted or unsubstituted condensed polycyclic aromatic group or a substituted or unsubstituted condensed polycyclic heterocyclic group, which may be identical or different from each other.)
Owner:CANON KK
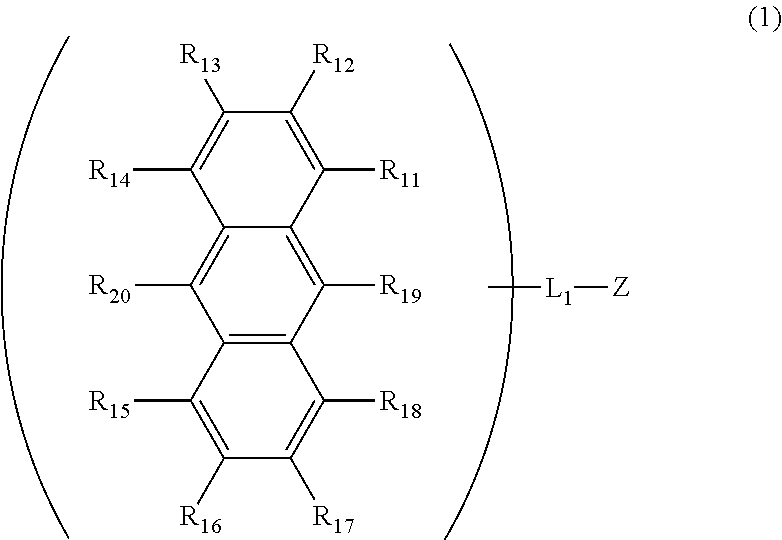
Anthracene derivative and organic electroluminescent element using same
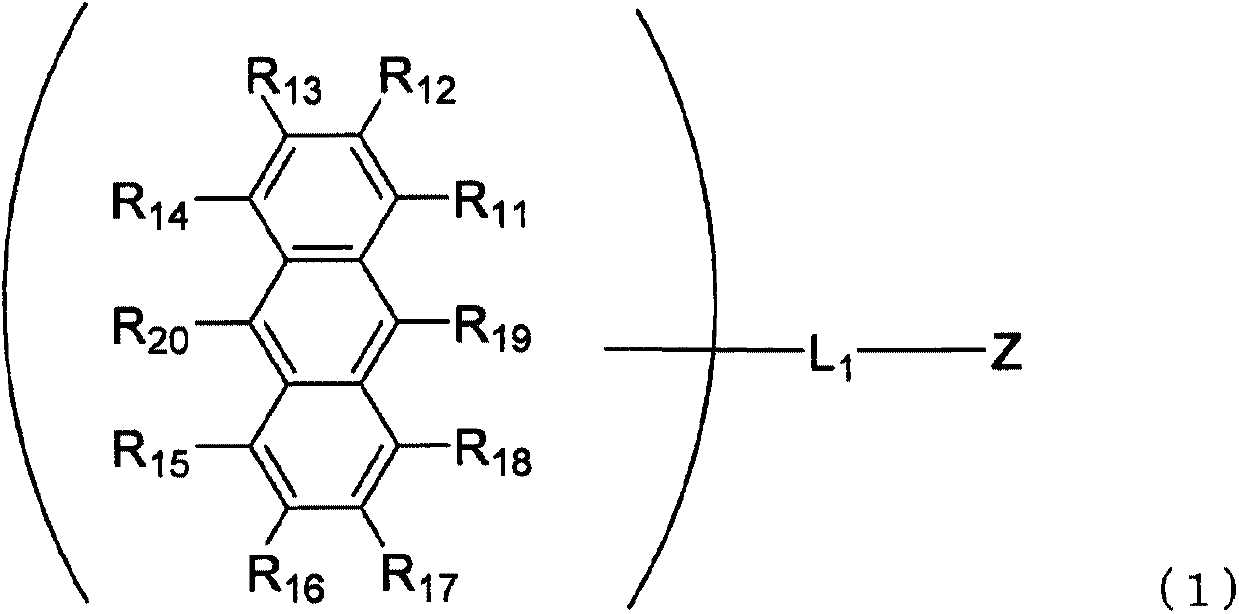
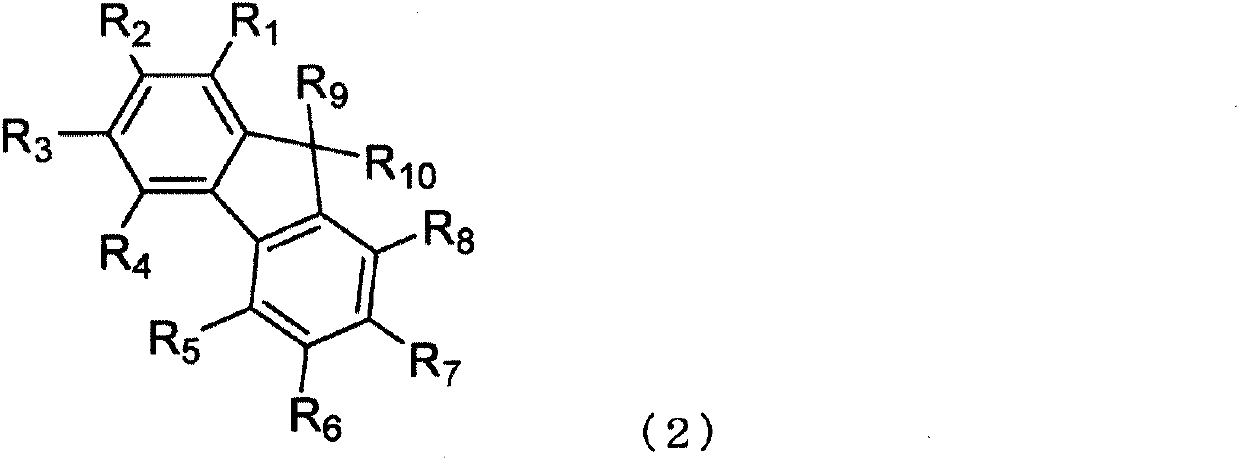
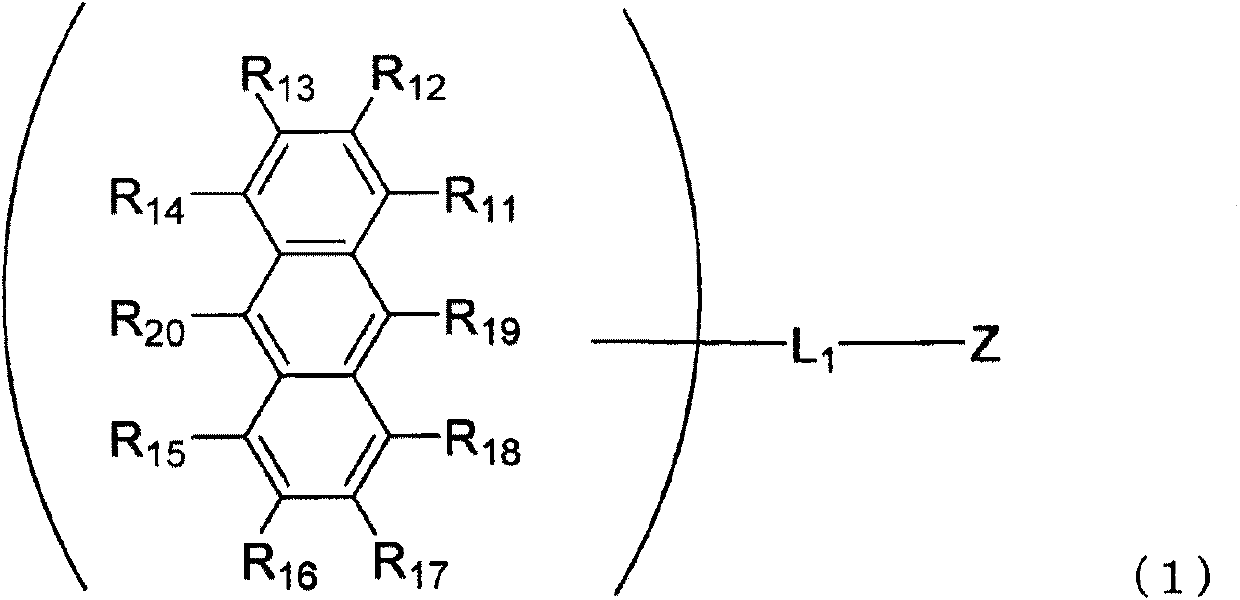
InactiveUS20160181542A1Solve low luminous efficiencyReduce voltageOrganic chemistrySolid-state devicesStructural formulaCarbon atom
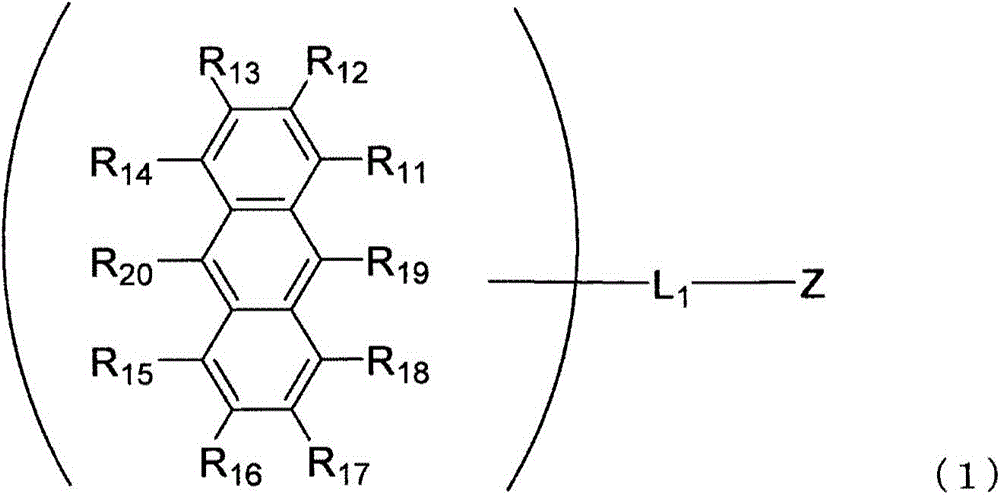
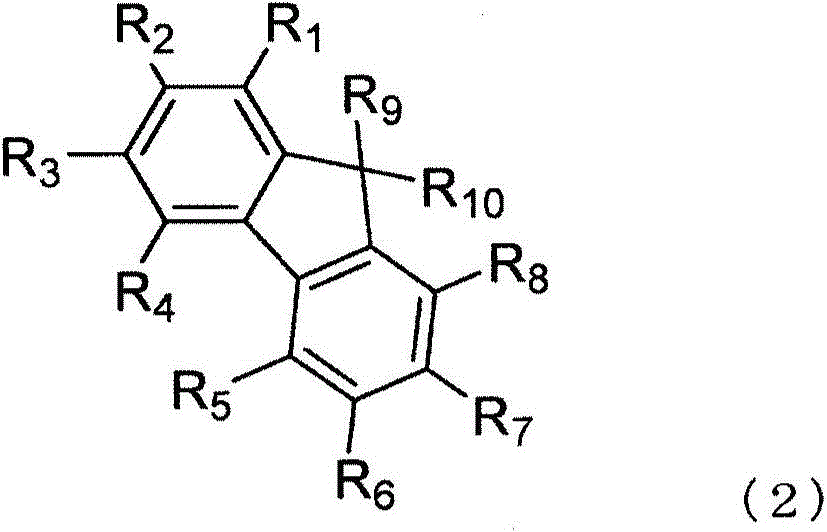
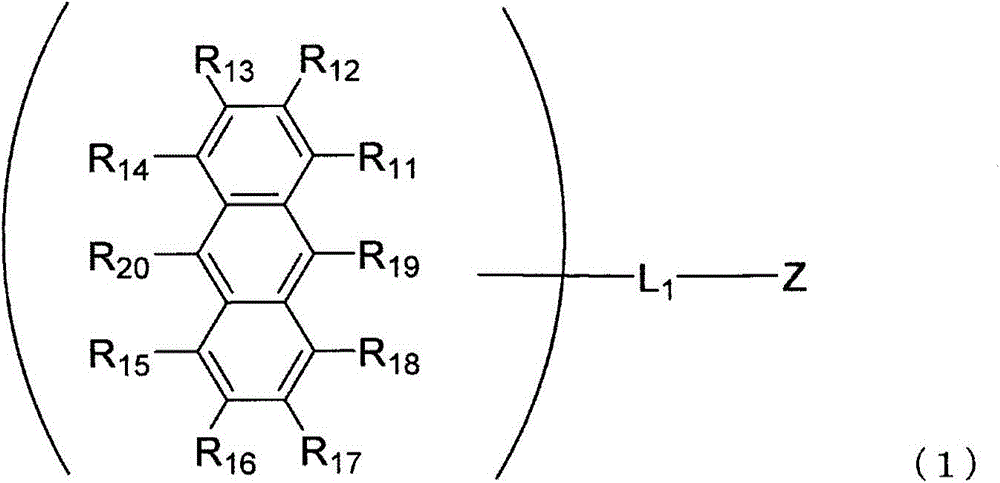
An anthracene derivative is represented by the following formula (1). In the formula (1), one of R11 to R20 is used to bond to L1, and is a single bond. The remainder of R11 to R20 that are not used to bond to L1 are independently a hydrogen atom, a halogen atom, a cyano group, a substituted or unsubstituted alkyl group including 1 to 20 carbon atoms, or the like. L1 is a single bond, a substituted or unsubstituted divalent aromatic hydrocarbon group including 6 to 50 ring carbon atoms, or the like. Z has a structure represented by the following formula (2). In the formula (2), one of R1, R3, and R4 is used to bond to L1, and is a single bond. The remainder of R1, R3, and R4 that are not used to bond to L1, R2, and R5 to R10 are independently a hydrogen atom, a halogen atom, a cyano group, a substituted or unsubstituted alkyl group including 1 to 20 carbon atoms, or the like. At least one pair of groups among R5 to R8 that are adjacent to each other are bonded to each other to form a saturated or unsaturated hydrocarbon ring.
Owner:IDEMITSU KOSAN CO LTD
Green Electroluminescent Compounds and Organic Electroluminescent Device Using the Same
InactiveUS20090128010A1Maximized electroluminescent efficiencyEfficient energy transfer mechanismOrganic chemistryDischarge tube luminescnet screensOrganic electroluminescenceLight-emitting diode
The present invention relates to organic electroluminescent compounds represented by Chemical Formula 1 or 2, a process for preparing the same, and an organic light emitting diode (OLED) which comprises, as a luminescent region interposed between an anode and a cathode, at least one compound (s) selected from those represented by Chemical Formula 1 or 2, and at least one compound selected from anthracene derivatives, benz[a]anthracene derivatives and naphthacene derivatives. The electroluminescent compound according to the present invention is a green electroluminescent compound having maximized electroluminescent efficiency and lifetime of device.
Owner:HYUN SEUNG HAK +10
Dyes of improved optical brightness and/or fluorescence and compositions containing them
Preparing dyes suitable for cosmetic use that start with known dyes and link them, for example, with 1,3,5 triazine to bulky organic groups that control solubility.
Owner:HOLMES ANDREA E
Superfine kaoline lamellar material preparation
InactiveCN1439677AHigh intercalation rateGood peeling effectBenzathrone dyesMaterials preparationPotassium acetate
A superfine kaolin flake material is prepared through mixing potassium acetate as sandwich agent with kaolin grinding, intercalating, laying aside, water washing, centrifugal separating of patassium acetate, stripping kaolin flakes, baking and grinding. Its advantages are high intercalating rate and slake-stripping effect, and low cost.
Owner:EAST CHINA NORMAL UNIVERSITY
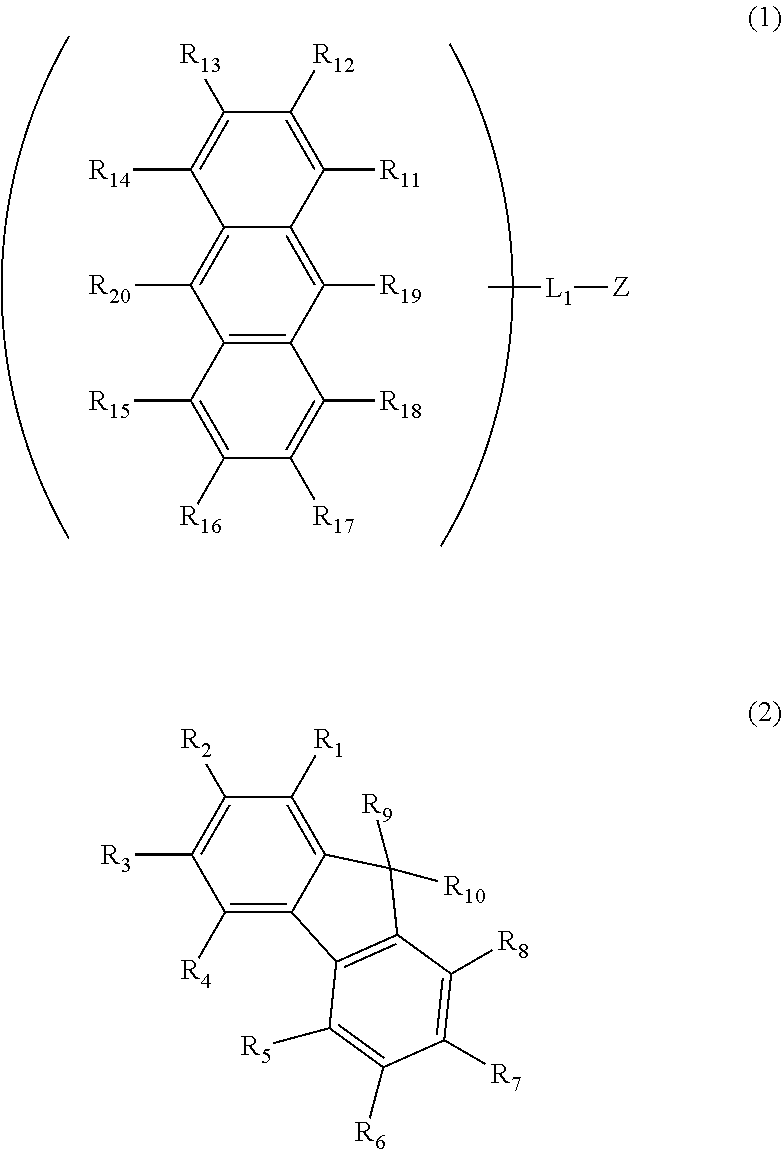
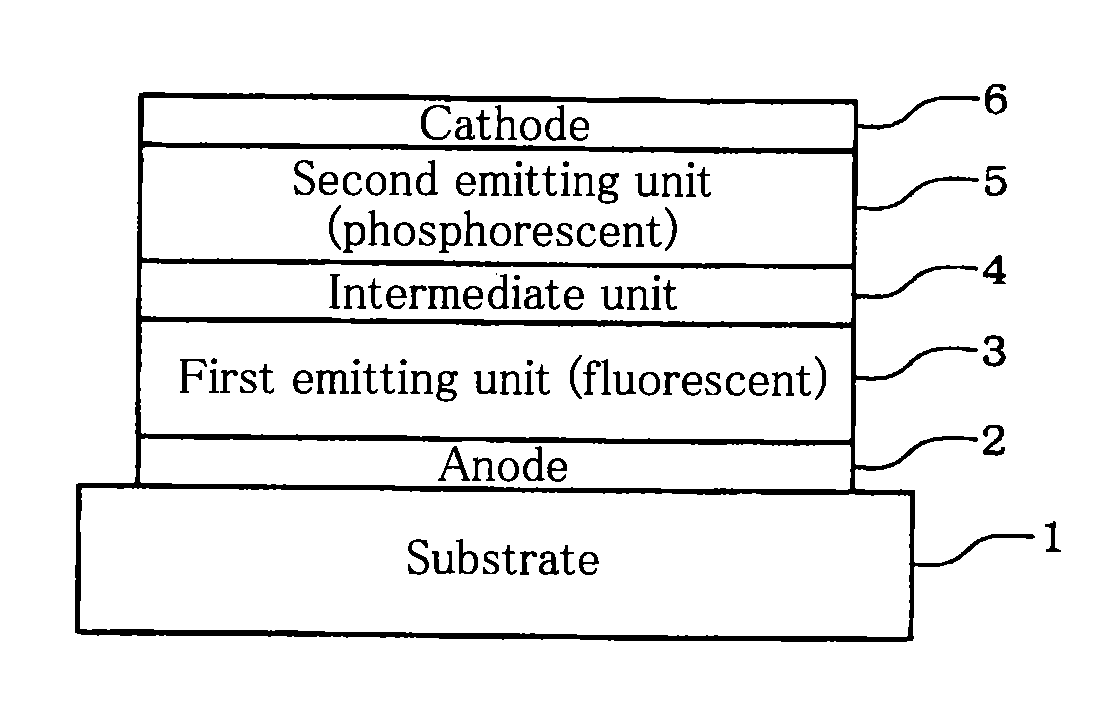
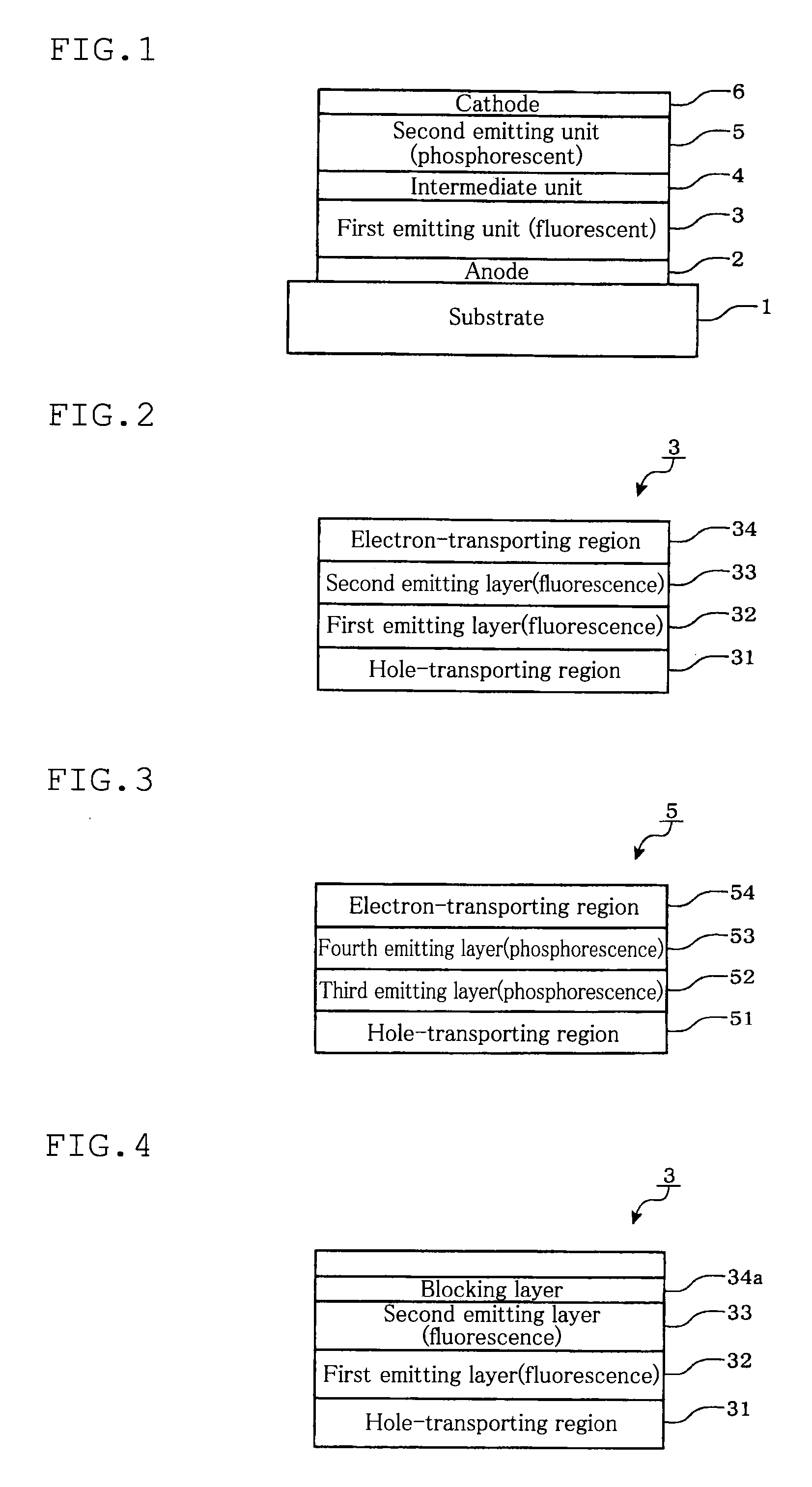
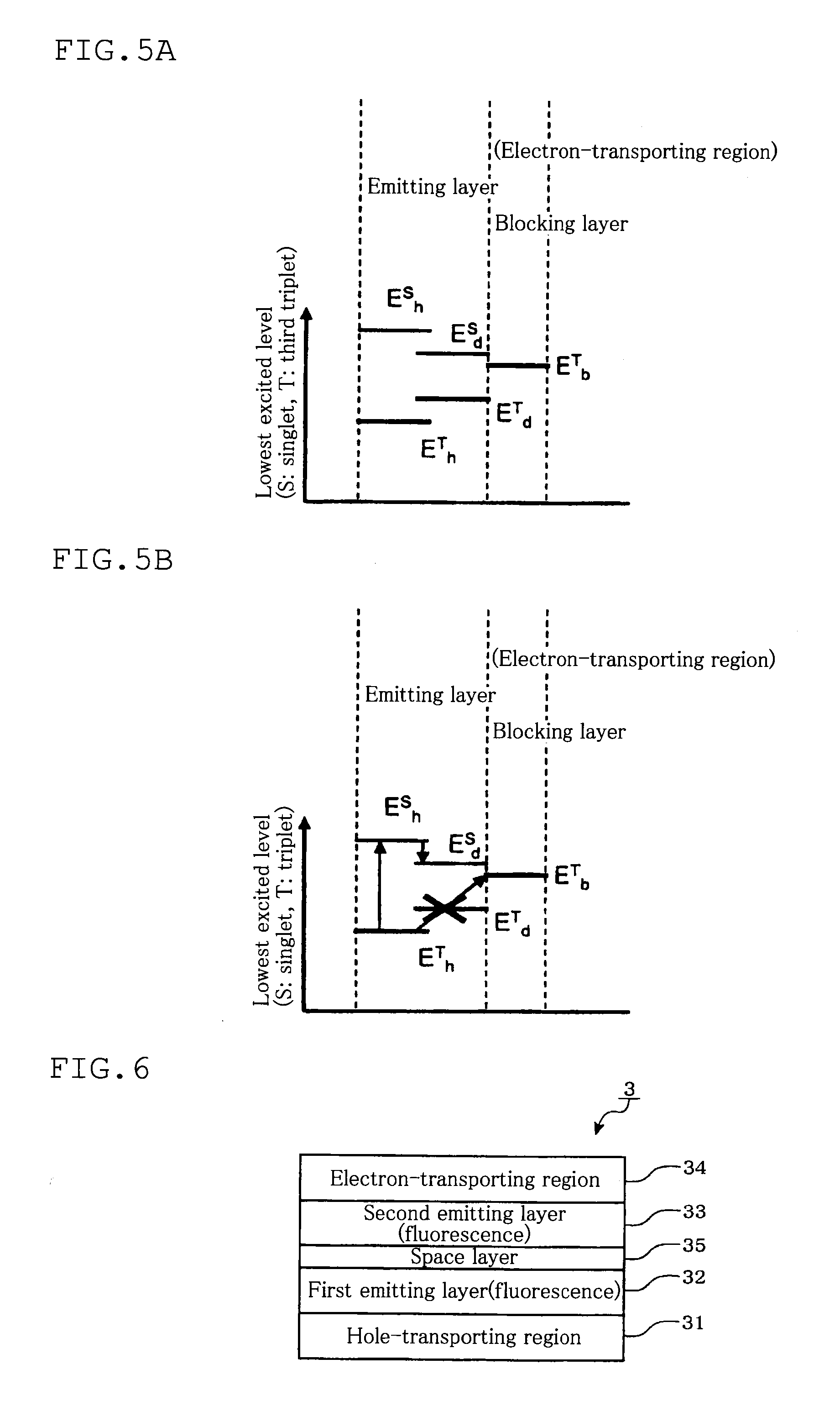
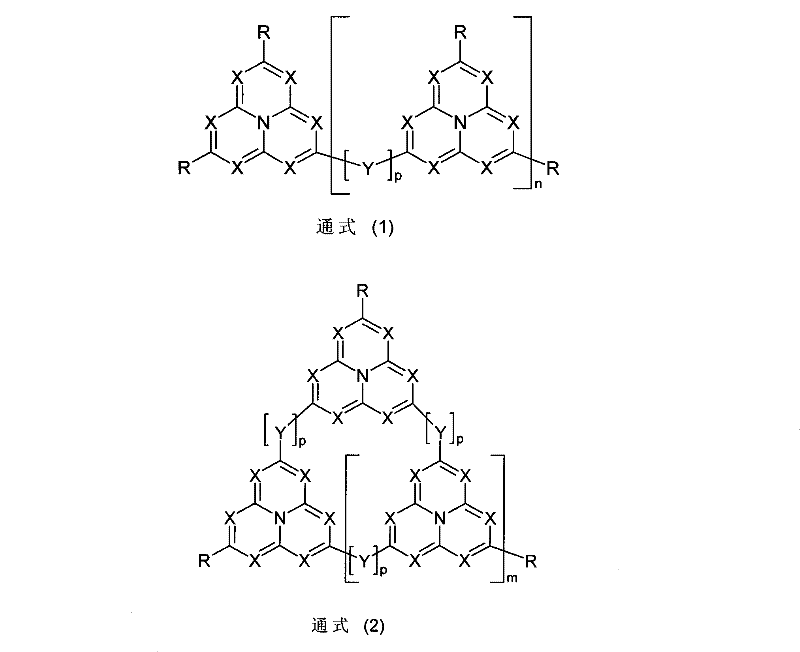
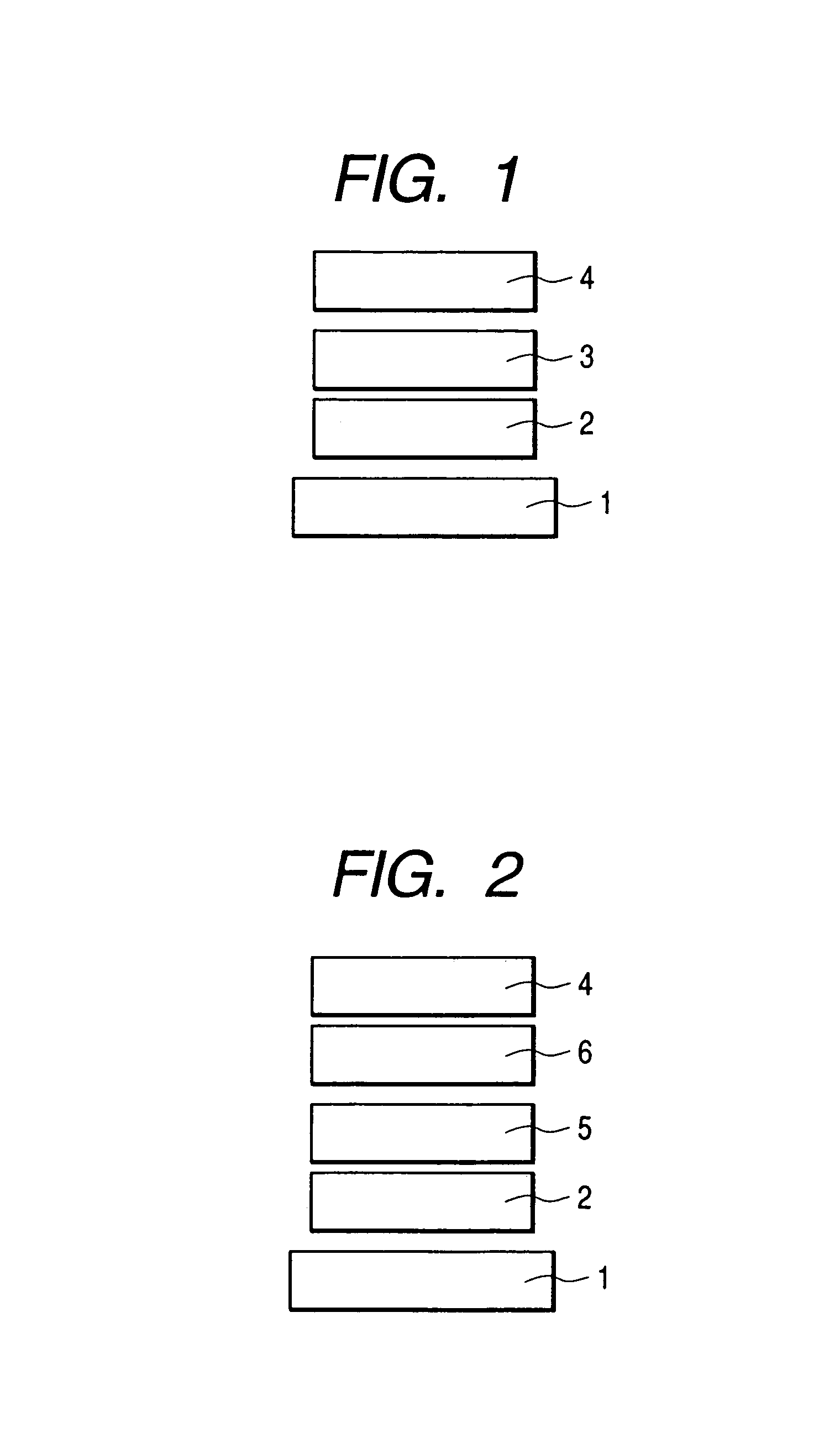
Tandem organic electroluminescence device
InactiveUS20130240859A1Solve low luminous efficiencySolution to short lifeElectroluminescent light sourcesSolid-state devicesDopantOrganic electroluminescence
A tandem organic electroluminescence device including a first emitting unit (3), an intermediate unit (4) and a second emitting unit (5) being stacked between opposing electrodes (2) and (6) the first emitting unit (3) including a blue fluorescent dopant and a red fluorescent dopant and the second emitting unit (5) including a red phosphorescent dopant and a green phosphorescent dopant.
Owner:IDEMITSU KOSAN CO LTD
Organic electronic device
ActiveCN102317408AImprove thermal stabilityImprove efficiencyOrganic chemistryElectroluminescent light sourcesHole injection layerNitrogenous heterocyclic compound
The present invention relates to organic electroluminescent devices comprising aromatic nitrogen heterocyclics, in particular in a hole injection layer and / or in a hole blocking layer and / or in an electron transport layer and / or in an emitting layer.
Owner:MERCK PATENT GMBH
Materials for Organic Electroluminescent Devices
The present invention relates to substituted benzo[c]phenanthrene derivatives and to the production and to the use thereof in electronic devices, and to the electronic devices themselves. The present invention relates in particular to benzo[c]phenanthrene derivatives substituted with at least one aromatic unit or at least one diarylamino unit.
Owner:MERCK PATENT GMBH
Anthracene derivative and organic electroluminescent element using same
ActiveCN105492413AImprove luminous efficiencyOrganic chemistrySolid-state devicesAnthraceneHydrogen atom
Provided is an anthracene derivative represented by formula (1). In formula (1), one of R11 to R20 is used to bond to L1, and those of R11 to R20 not used to bond to L1 are each independently a hydrogen atom, a halogen atom, a cyano group, a substituted or unsubstituted alkyl group having 1-20 carbon atoms, or the like. L1 is a single bond, a substituted or unsubstituted ring-forming divalent aromatic hydrocarbon group having 6-50 carbon atoms, or the like. Z is a structure represented by formula (2). In formula (2), one of R1, R3 and R4 is used to bond to L1, and those of R1, R3 and R4 not used to bond to L1, R2 and R5 to R10 are each independently a hydrogen atom, a halogen atom, a cyano group, a substituted or unsubstituted alkyl group having 1-20 carbon atoms, or the like. However, among R5 to R8, at least one pair of two adjacent groups bond to each other to form a saturated or unsaturated hydrocarbon ring.
Owner:IDEMITSU KOSAN CO LTD
Green electroluminescent compounds and organic electroluminescent device using the same
InactiveCN101243157AOrganic chemistryOrganic compound preparationAnthraceneOrganic electroluminescence
Owner:GRACEL DISPLAY INC
Novel materials for organic electroluminescent devices
ActiveCN101679855AOrganic chemistryElectroluminescent light sourcesHost materialOrganic electroluminescence
Owner:MERCK PATENT GMBH
Spiro compound and organic luminescence device using the same
InactiveUS7510781B2Improve efficiencyIncrease brightnessMethine/polymethine dyesSolid-state devicesArylHalogen
Provided are a novel spiro compound, and an organic luminescence device using the spiro compound and having an optical output with an extremely high efficiency and a high luminance, and an extremely high durability. The Spiro compound is represented by the following general formula [I]:(wherein R1, R2, R3, and R4 represent a hydrogen atom, a substituted or unsubstituted alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted heterocyclic group, a substituted amino group, a cyano group, or a halogen atom, and R1, R2, R3, and R4 may be identical or different from each other; and Ar1 and Ar2 represent a substituted or unsubstituted condensed polycyclic aromatic group or a substituted or unsubstituted condensed polycyclic heterocyclic group, which may be identical or different from each other.)
Owner:CANON KK
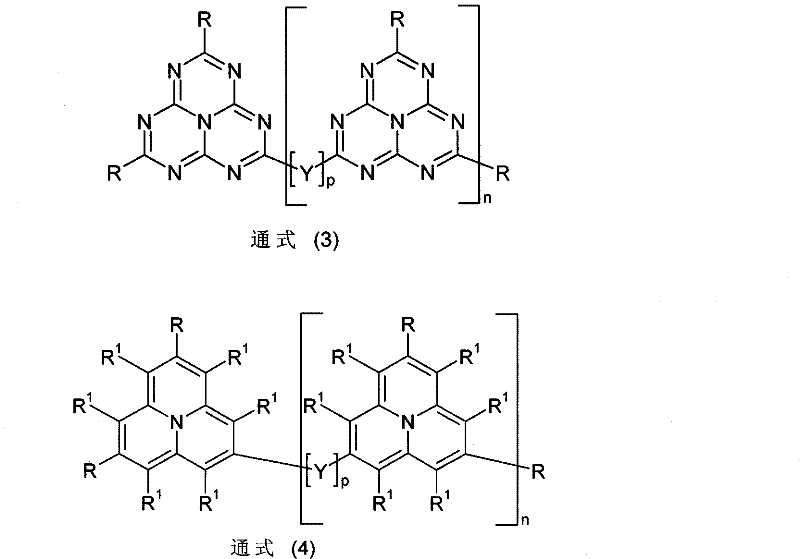
Pyrene polymer compound and light emitting device using the same
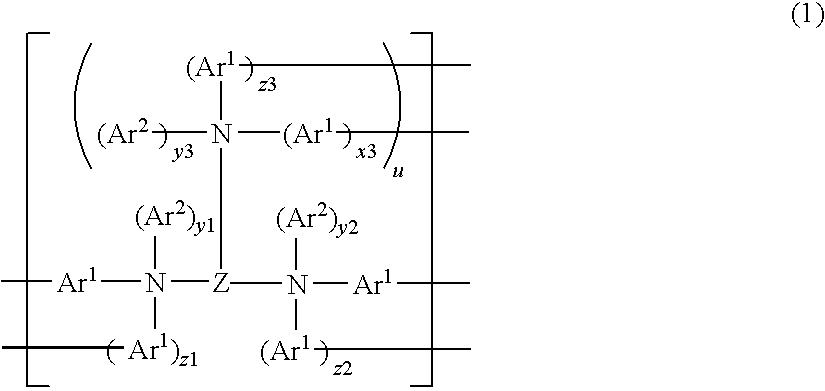
InactiveUS20100109517A1Improve balanceLarge thicknessOrganic chemistryDischarge tube luminescnet screensPolymer scienceLight emitting device
A polymer compound comprising a constitutional unit of the formula (1), and a repeating unit of any of the formulae (2) to (4):(wherein, x3, y1, y2, z1 to z3 are 0 or 1. y3 represents an integer of 0 to 2. u represents an integer of 0 to 8. Z represents a pyrene residue, Ar1 represents an arylene group or di-valent heterocyclic group, and Ar2 represents an aryl group or mono-valent heterocyclic group.)
Owner:SUMITOMO CHEM CO LTD
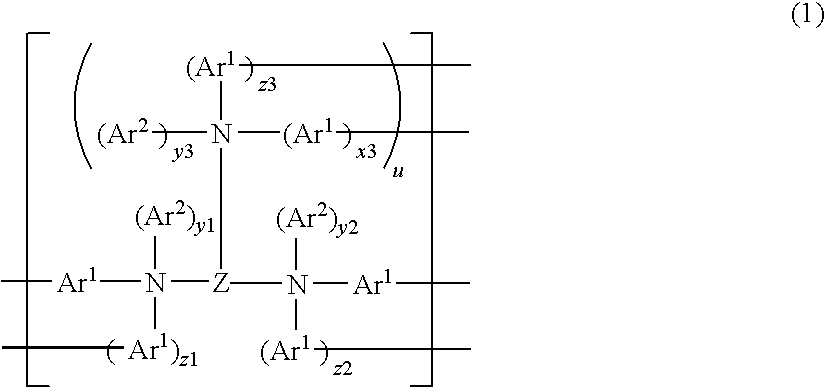
Functional fluorescent dyes
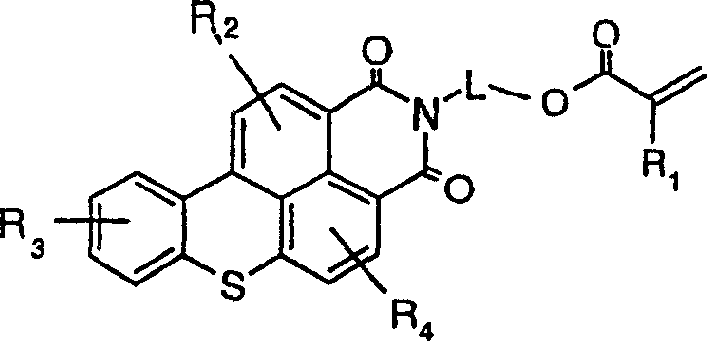
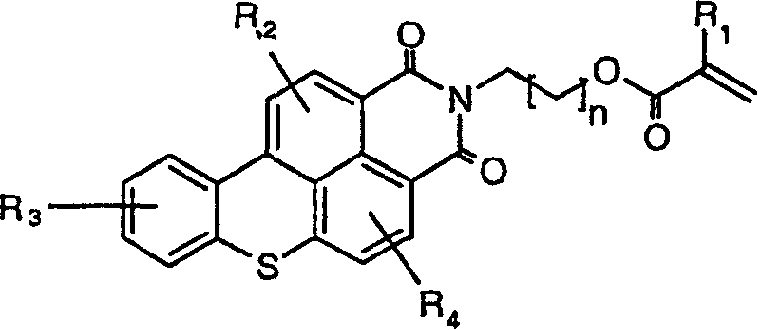
Fluorescent functional dyes represented by the following formulas: wherein R1 is hydrogen or methyl; R2, R3, R4, R5, and R6 are each independently selected from the group consisting of hydrogen, alkyl, aryl, alkoxy, aralkyl, alkaryl, halo, and trifluoromethyl; and L is a straight chain or branched chain alkylene containing 3 to about 15 carbon atoms, the alkylene group optionally containing one or more catenary heteroatoms.
Owner:3M INNOVATIVE PROPERTIES CO
Dyes of improved optical brightness and/or fluorescence and compositions containing them
Preparing dyes suitable for cosmetic use that start with known dyes and link them, for example, with 1,3,5 triazine to bulky organic groups that control solubility.
Owner:HOLMES ANDREA E
Benzanthracene derivatives for organic electroluminescent devices
The present invention relates to the compounds of the formula (1) and to organic electroluminescent devices, in particular blue-emitting devices, in which these compounds are used as host material or dopant in the emitting layer and / or as hole-transport material and / or as electron-transport material.
Owner:MERCK PATENT GMBH
Benzanthracene derivative and electroluminescence device using the same
ActiveUS7829206B2Improve efficiencyGood chromaSolid-state devicesSemiconductor/solid-state device manufacturingOrganic filmOrganic electroluminescence
A benzanthracene derivative having hydrogen atom at the 12-position, and an organic electroluminescence device having an organic thin film layer, which has one layer or a plurality of layers including at least a light emitting layer, is disposed between a cathode and an anode and contains the benzanthracene derivative in at least one layer in the organic thin film layer singly or as a component of a mixture. The electroluminescence device provides a great efficiency of light emission, has a long life and exhibits an excellent chromaticity.
Owner:IDEMITSU KOSAN CO LTD
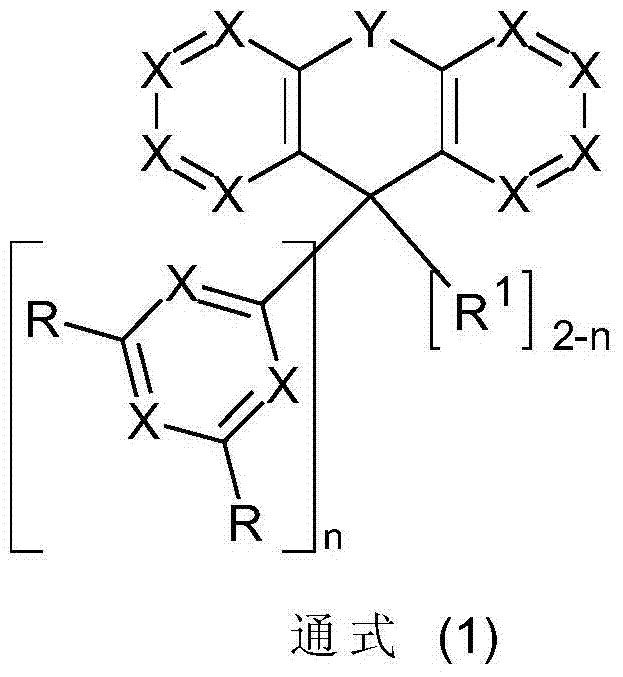
Materials for electronic devices
InactiveCN102791659AImprove efficiencyExtend your lifeOrganic compound preparationGroup 5/15 element organic compoundsDopantHost material
The present invention relates to compounds according to the general formula (I), the use thereof in electronic devices, preferably as a host material for fluorescent dopants or as a fluorescent dopant, to methods for producing the compounds according to formula (I), and to electronic devices containing compounds according to formula (I).
Owner:MERCK PATENT GMBH
Organic electronic device
ActiveUS9066410B2Improve thermal stabilityImprove efficiencyOrganic chemistryElectroluminescent light sourcesHole injection layerNitrogen
The present invention relates to organic electroluminescent devices which comprise aromatic nitrogen heterocyclic compounds, in particular in a hole-injection layer and / or in a hole-blocking layer and / or in an electron-transport layer and / or in an emitting layer.
Owner:MERCK PATENT GMBH
Fluorine derivatives for organic electroluminescence devices
ActiveCN104327003AImprove thermal stabilityImprove efficiencyGroup 5/15 element organic compoundsSolid-state devicesHost materialOrganic electroluminescence
The present invention relates to fluorine derivatives and organic electronic devices in which said compounds are used as a matrix material in the emitting layer and / or as a hole transport material, and / or as an electron blocking or exciton blocking material, and / or as an electron transport material.
Owner:MERCK PATENT GMBH
Superfine kaoline lamellar material preparation
InactiveCN100404626CHigh intercalation rateGood peeling effectBenzathrone dyesMaterials preparationPotassium acetate
Owner:EAST CHINA NORMAL UNIV
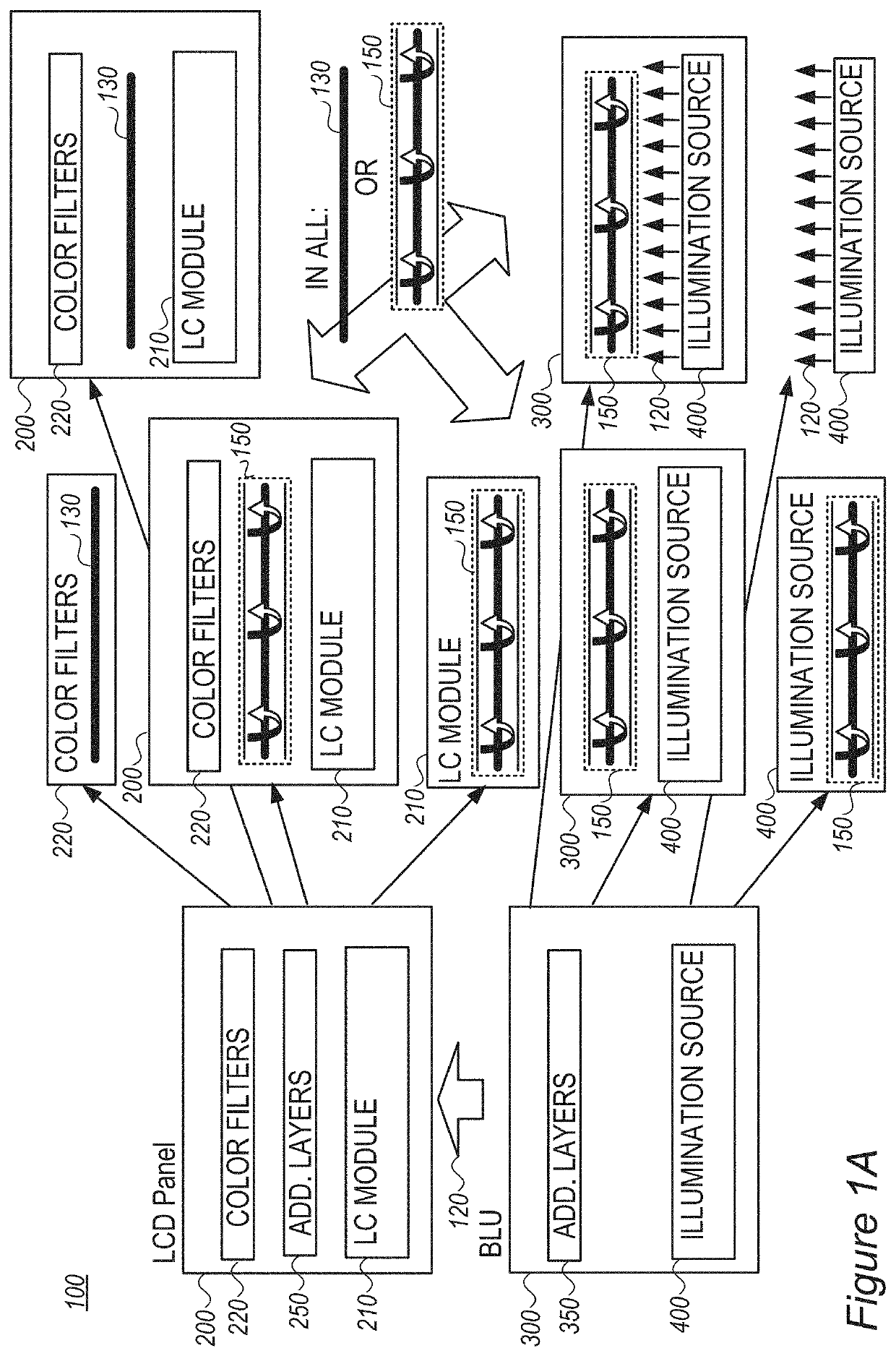
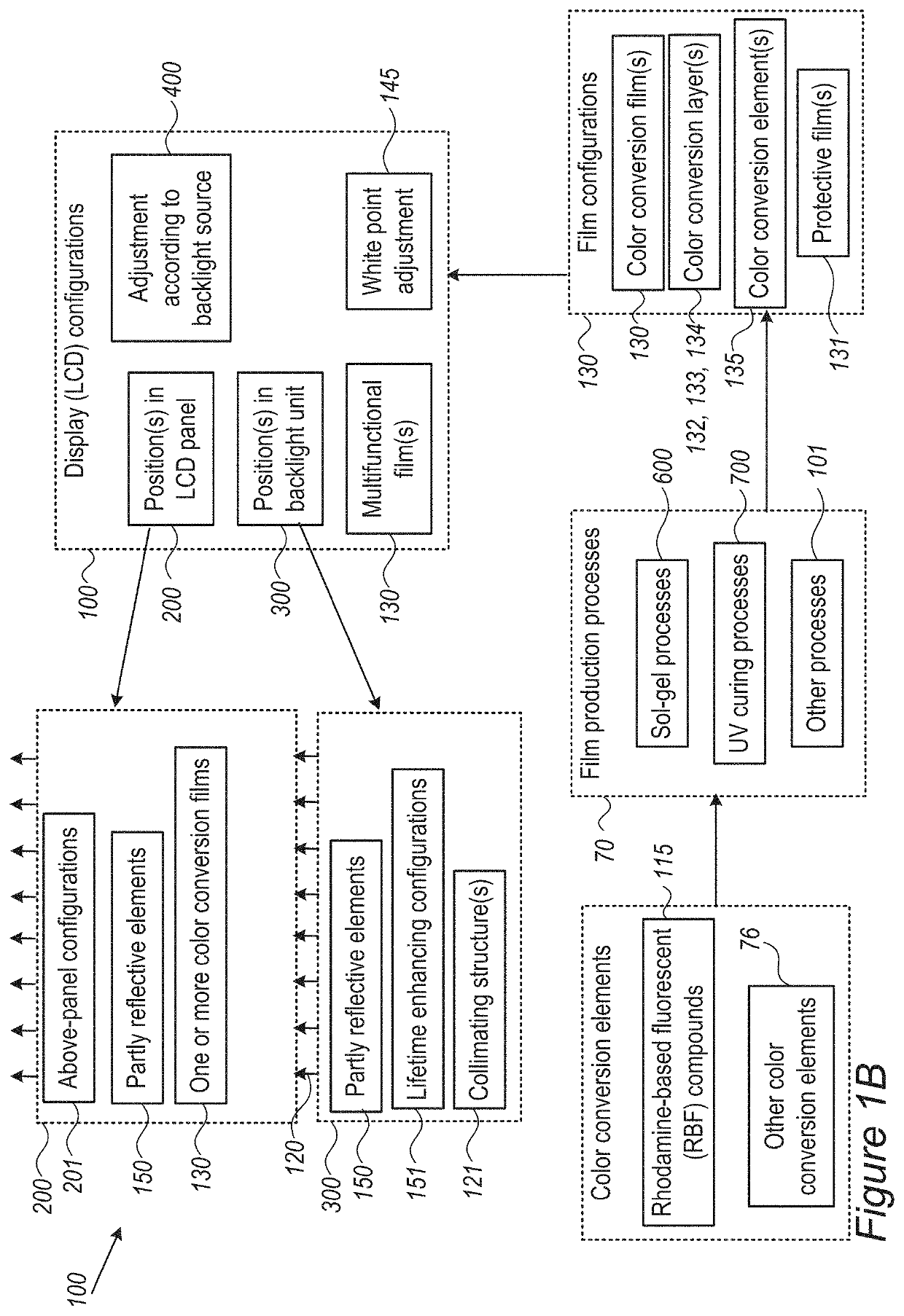
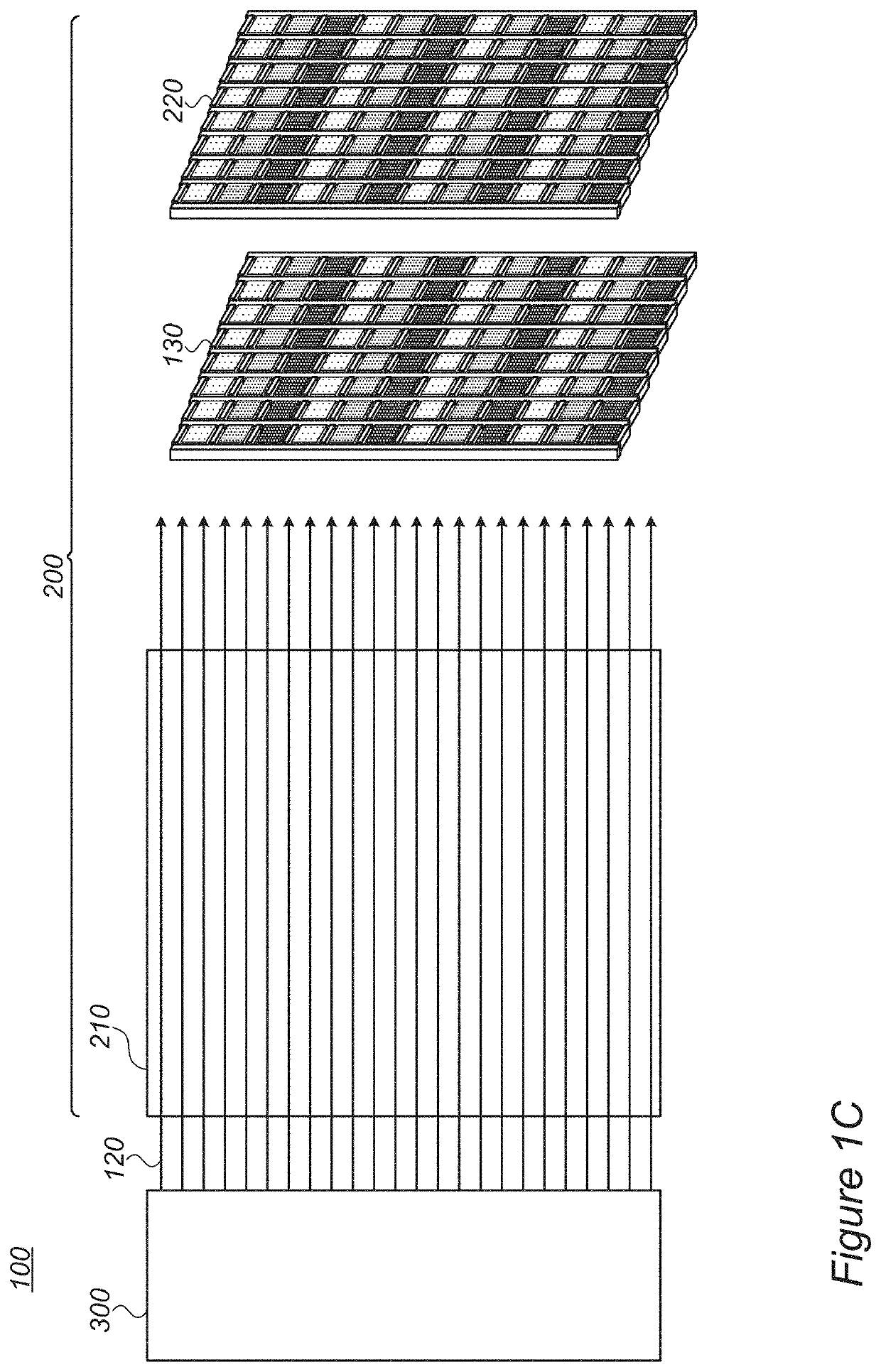
Assistant dyes for color conversion in LCD displays
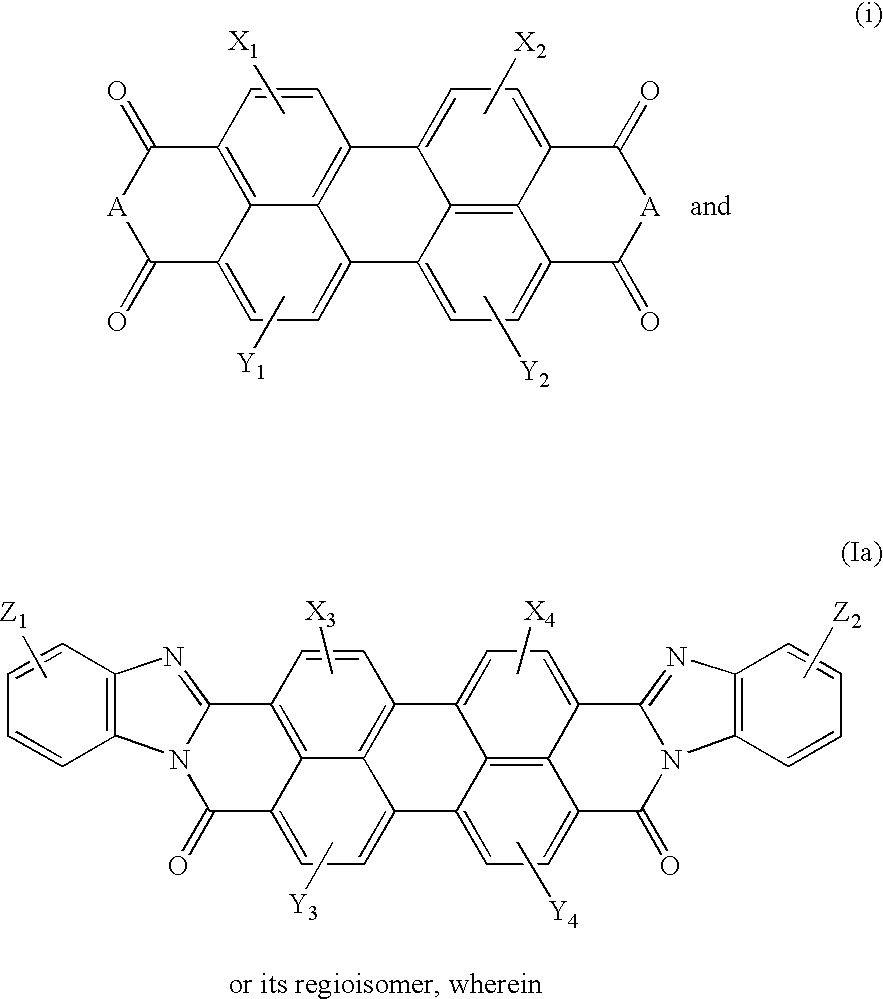
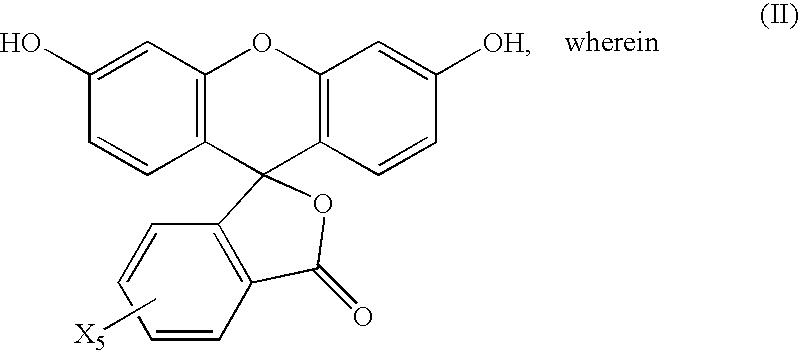
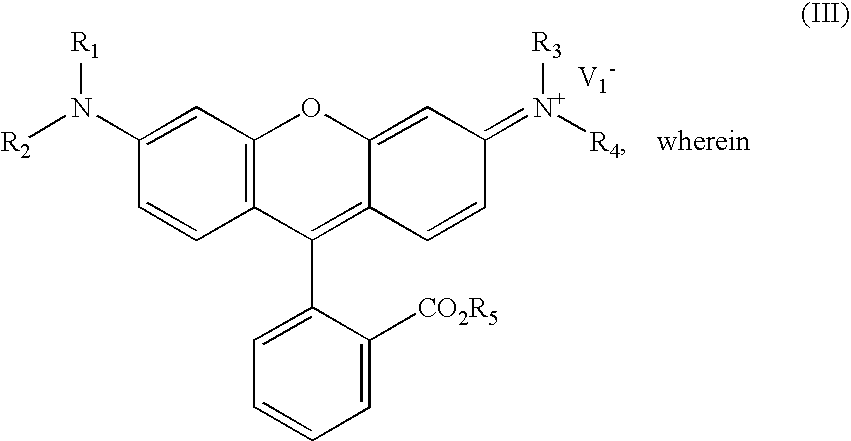
InactiveUS20210191198A1Limit scopeOrganic chemistryBenzathrone dyesColor transformationLiquid-crystal display
LCDs (liquid crystal displays) with improved efficiency and performance, as well as corresponding methods are disclosed. Color conversion films and elements with rhodamine-based fluorescent compounds and / or assistant dyes are used to modify the spectrum of the illumination provided by the backlight unit in either or both the backlight unit itself and the LCD panel, in various configurations. Color conversion may be performed above the LC module, possibly by a patterned layer incorporating the color filters, and / or within the backlight unit within a fluorescence-intensifying section in which radiation is recycled to enhance color conversion. Film configuration, positions and optionally supportive structures are provided, to extend the lifetime of the fluorescent compounds. Collimation of backlight illumination may further enhance the optical performance of disclosed LCDs.
Owner:MOLECULED LTD
Organic electroluminescent element and lighting device
ActiveUS9184414B2Improve light extraction efficiencyImprove external quantum efficiencySemiconductor/solid-state device detailsElectroluminescent light sourcesEffect lightRefractive index
An organic EL device comprises: a light-transmissive substrate; a transparent electrode; an organic emitting layer; and an opposing electrode. A light-extraction layer provided by layering a high refractive index layer adjoining the transparent electrode and a low refractive index layer adjoining the light-transmissive substrate includes recess-protrusion units each provided by a protrusion and a recess at an interface between the high refractive index layer and the low refractive index layer. In at least one of the recess-protrusion units, a distance from the interface between the high refractive index layer and the low refractive index layer to an interface between the transparent electrode and the high refractive index layer is equal to or more than an optical coherence length and a height of the protrusion, a width of the protrusion and a gap between the protrusion and another protrusion adjoining the protrusion across the recess is 1 μm or more.
Owner:IDEMITSU KOSAN CO LTD
Novel organic dye and method for preparing same
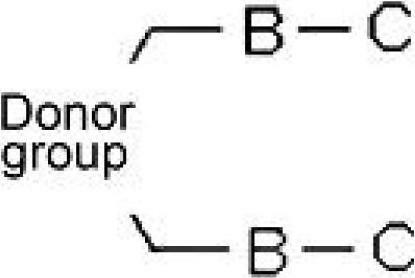
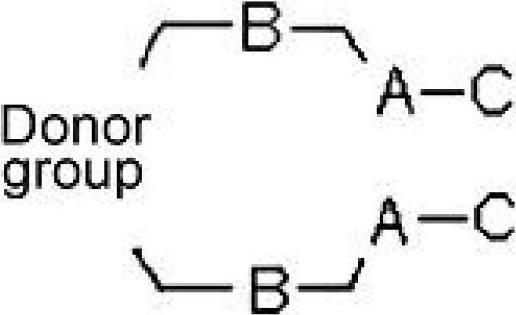
InactiveCN102695759ALower synthesis costImprove efficiencyMonoazo dyesAcridine dyesOrganic dyeElectron donor
The present invention relates to novel organic dye and method for preparing the same. A dye compound of the present invention comprises: a specific aliphatic compound as an electron donor; and a thiopheneic or dehydrothiopheneic unit in an intermediate connecting part (spacer). The dye compound of the present invention is used in a dye-sensitized solar cell (DSSC) to improve a molar extinction coefficient, Jsc (short circuit photocurrent density) and photoelectric conversion efficiency as compared with those of conventional dyes, and thus significantly improves the efficiency of the solar cell. The dye compound of the present invention can be refined without using expensive columns, thereby remarkably reducing costs for dye synthesis.
Owner:DONGJIN SEMICHEM CO LTD
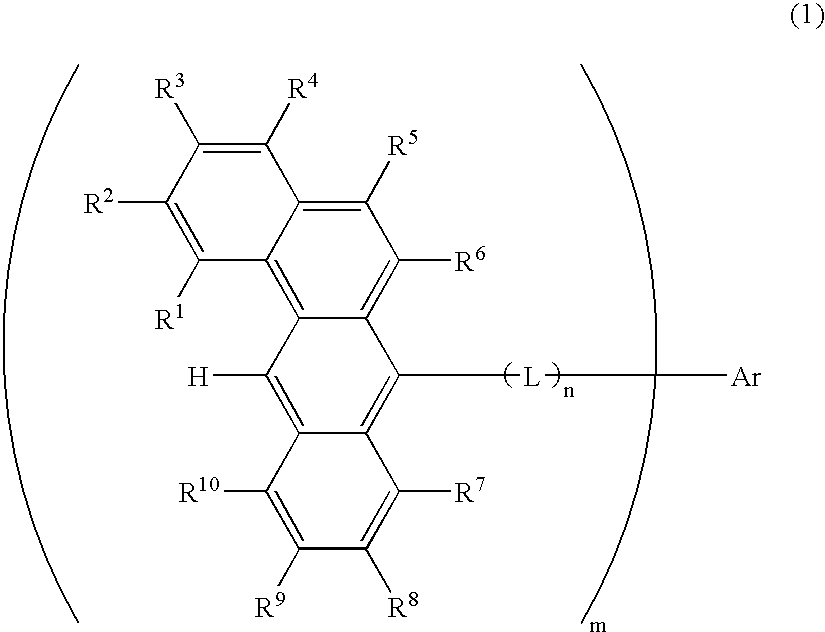
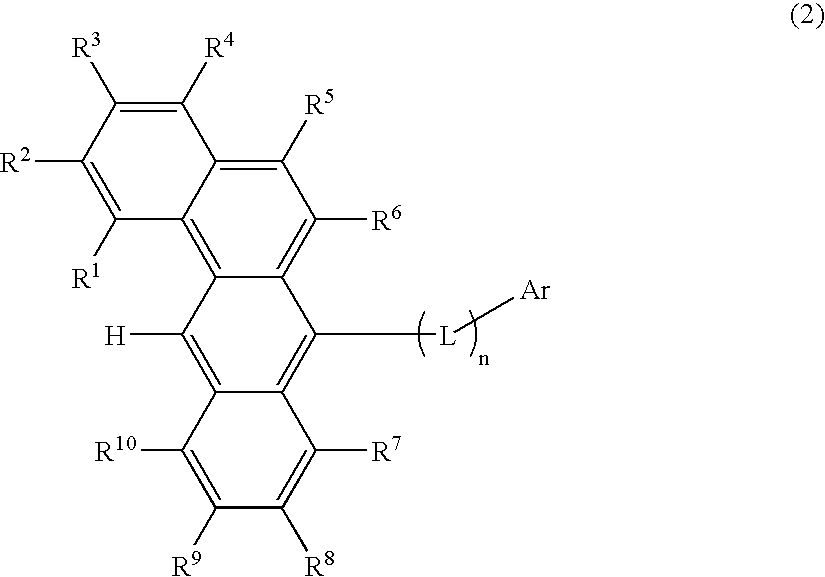
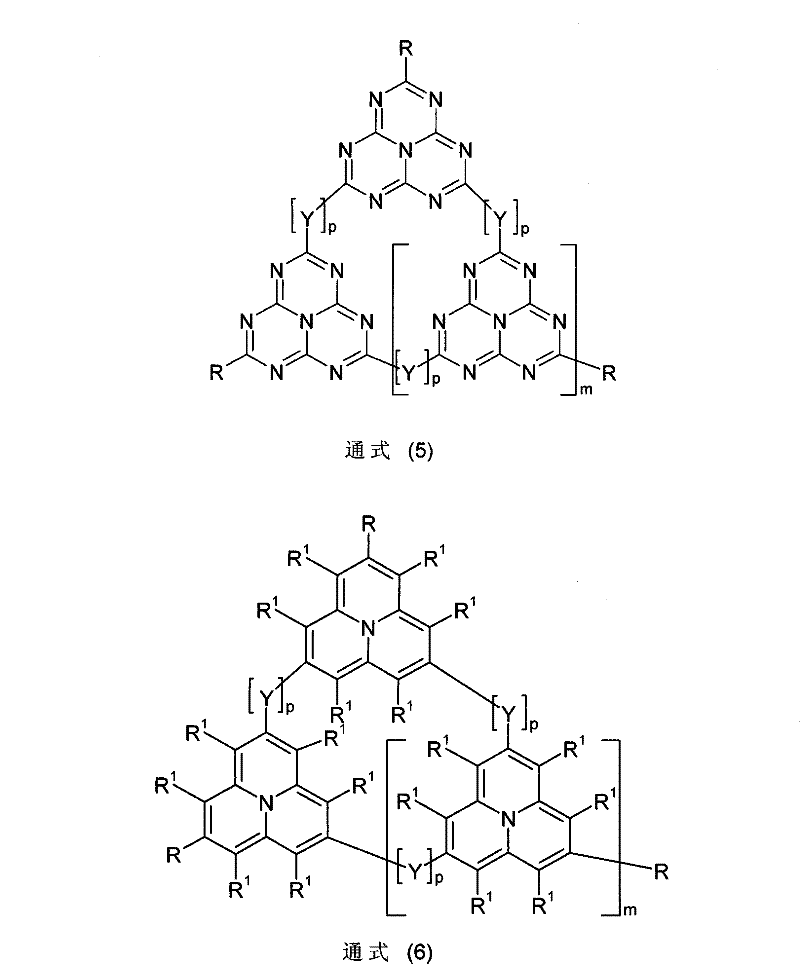
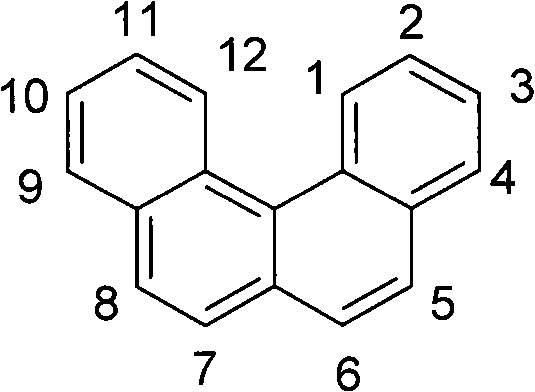
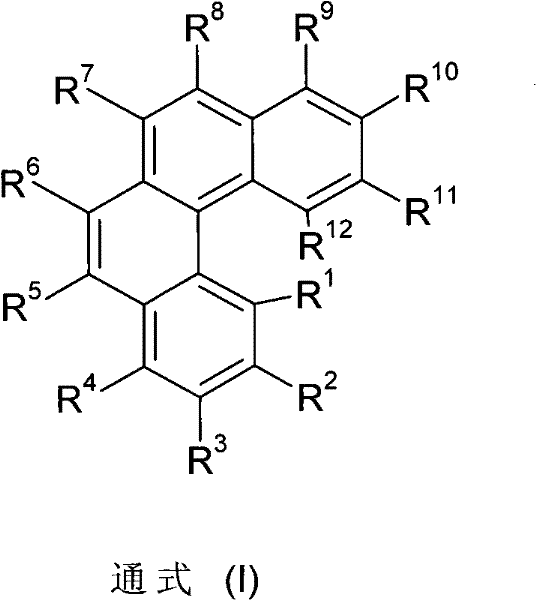
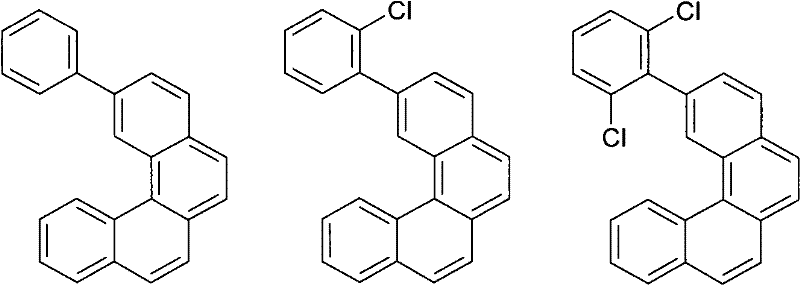
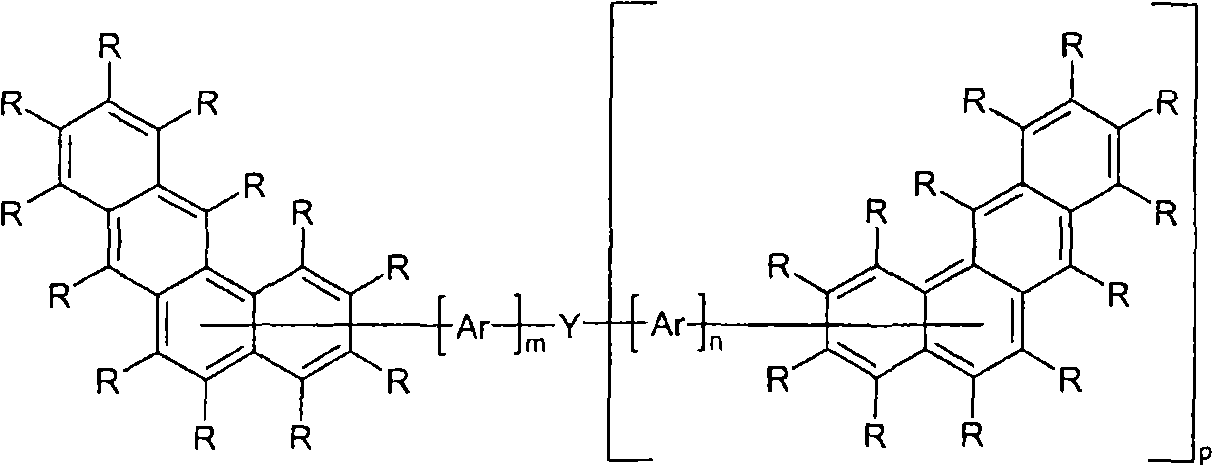
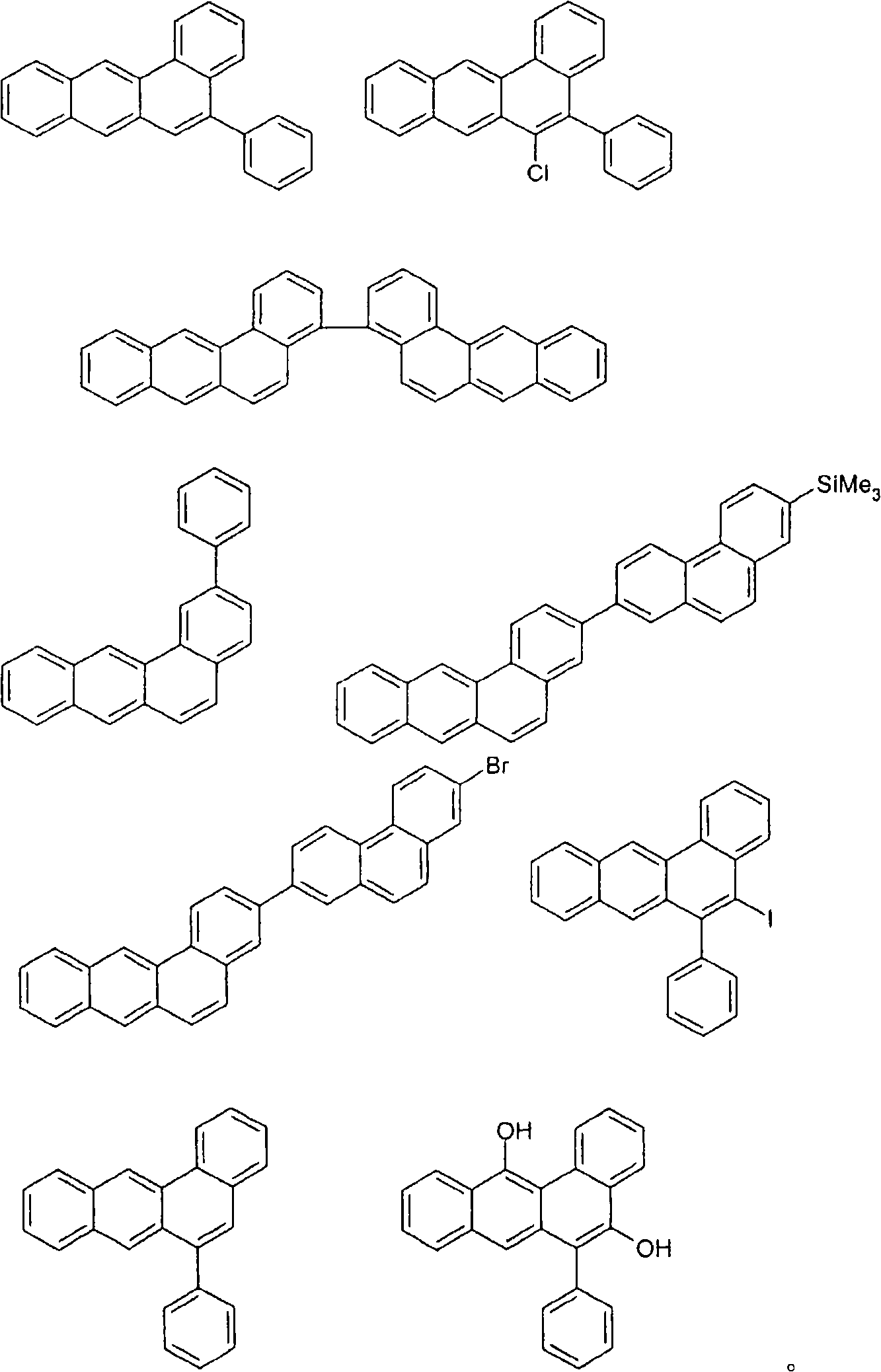
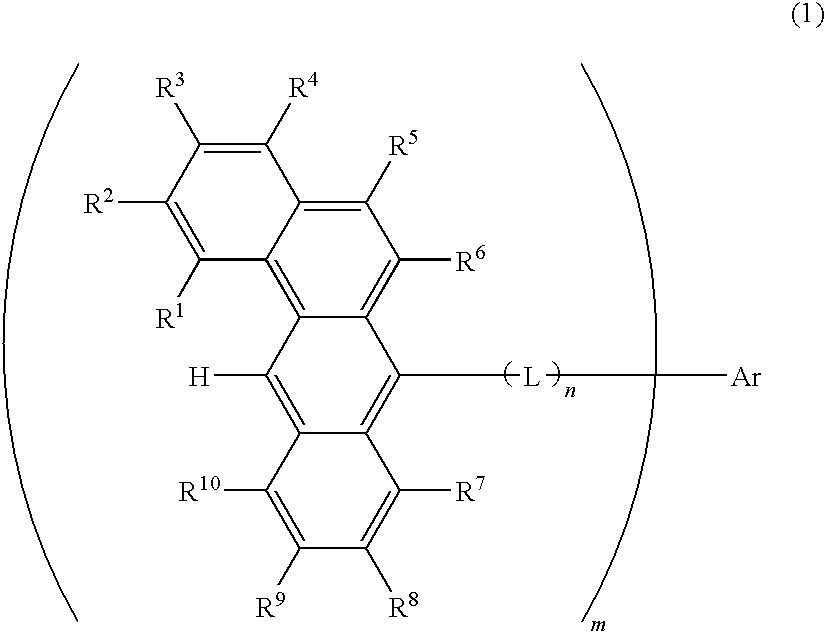
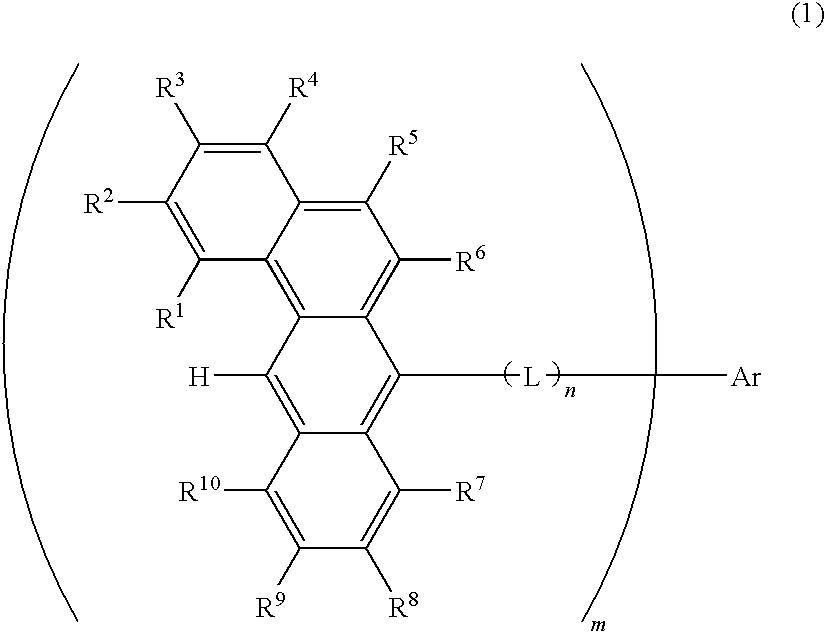
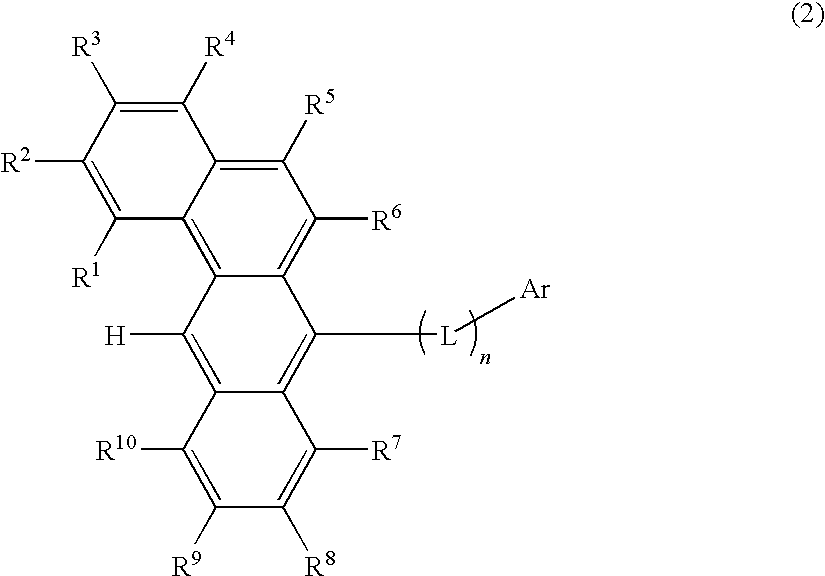
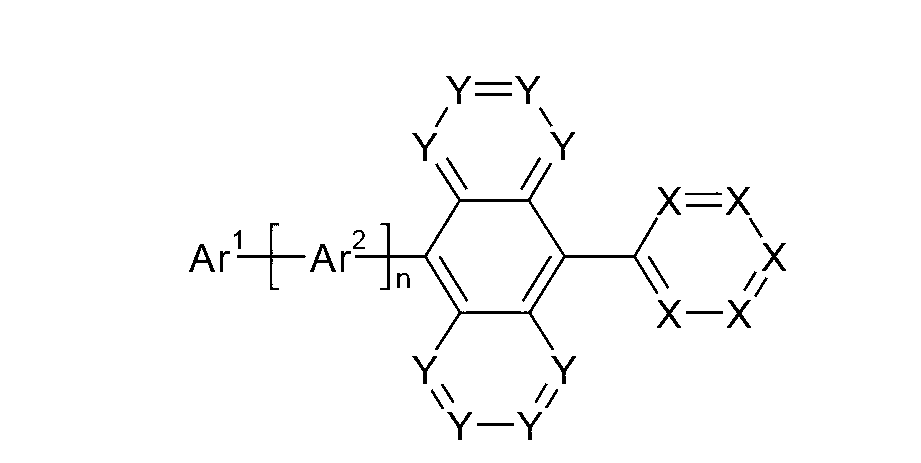
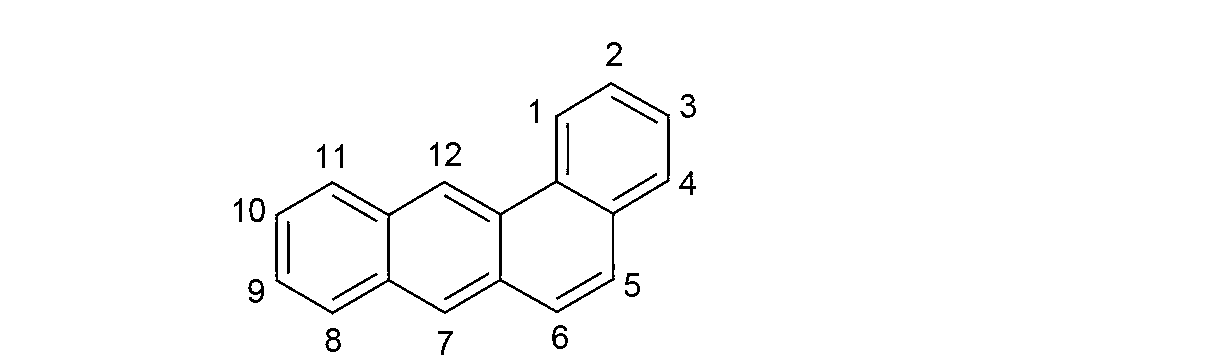
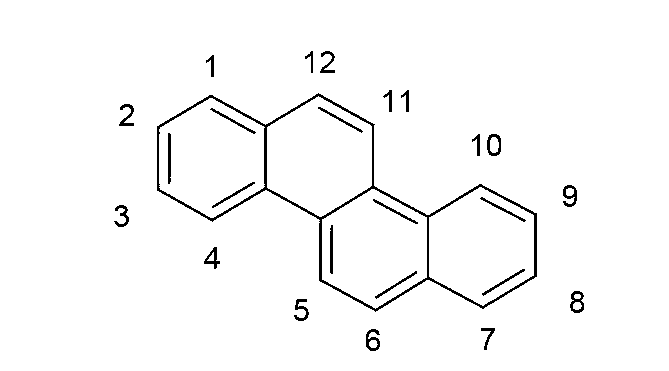
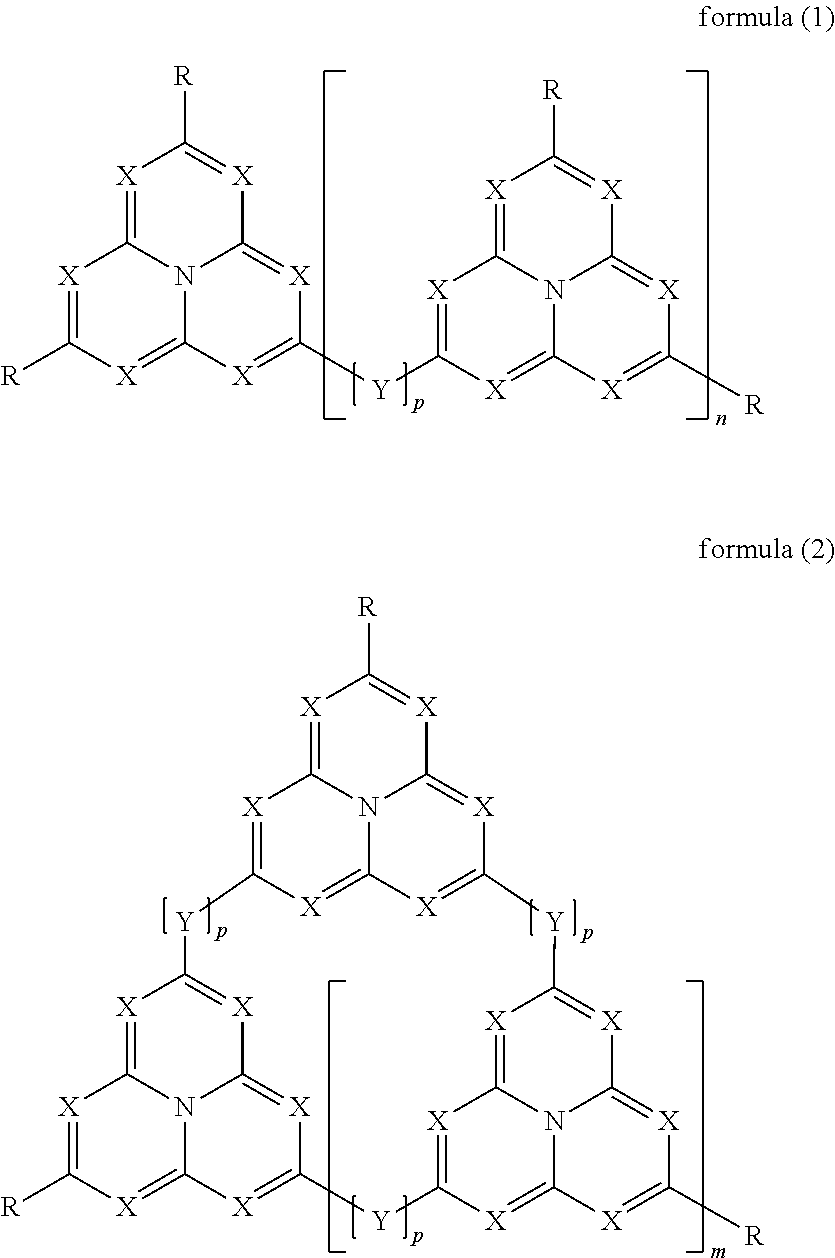
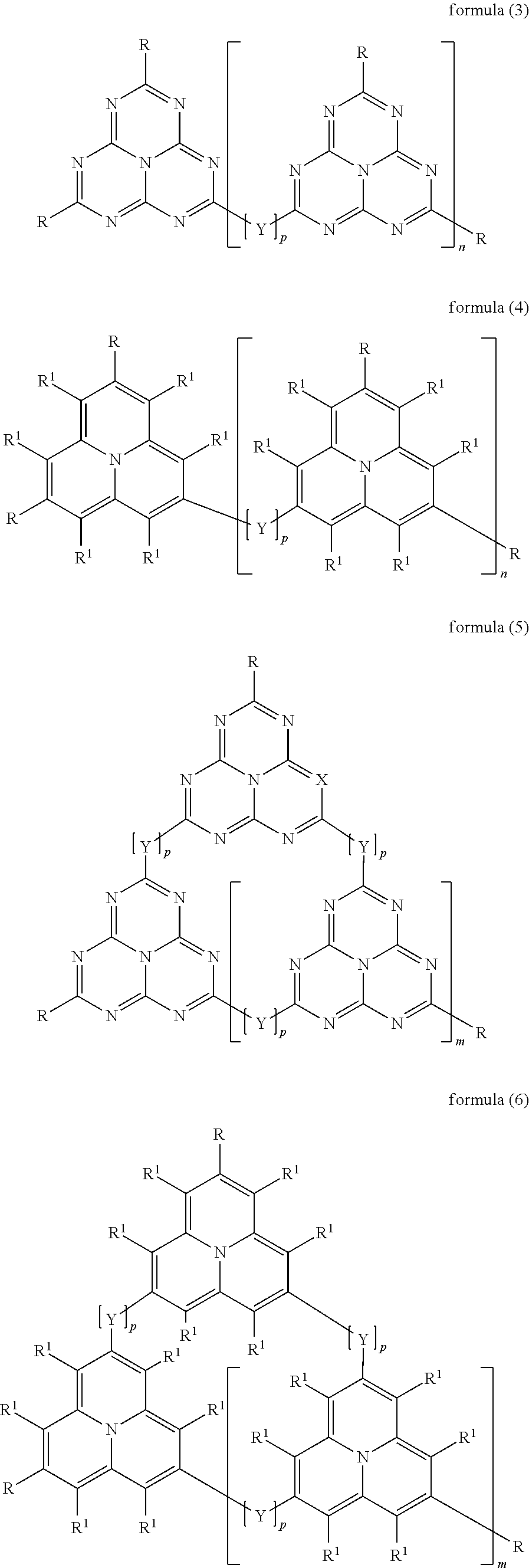
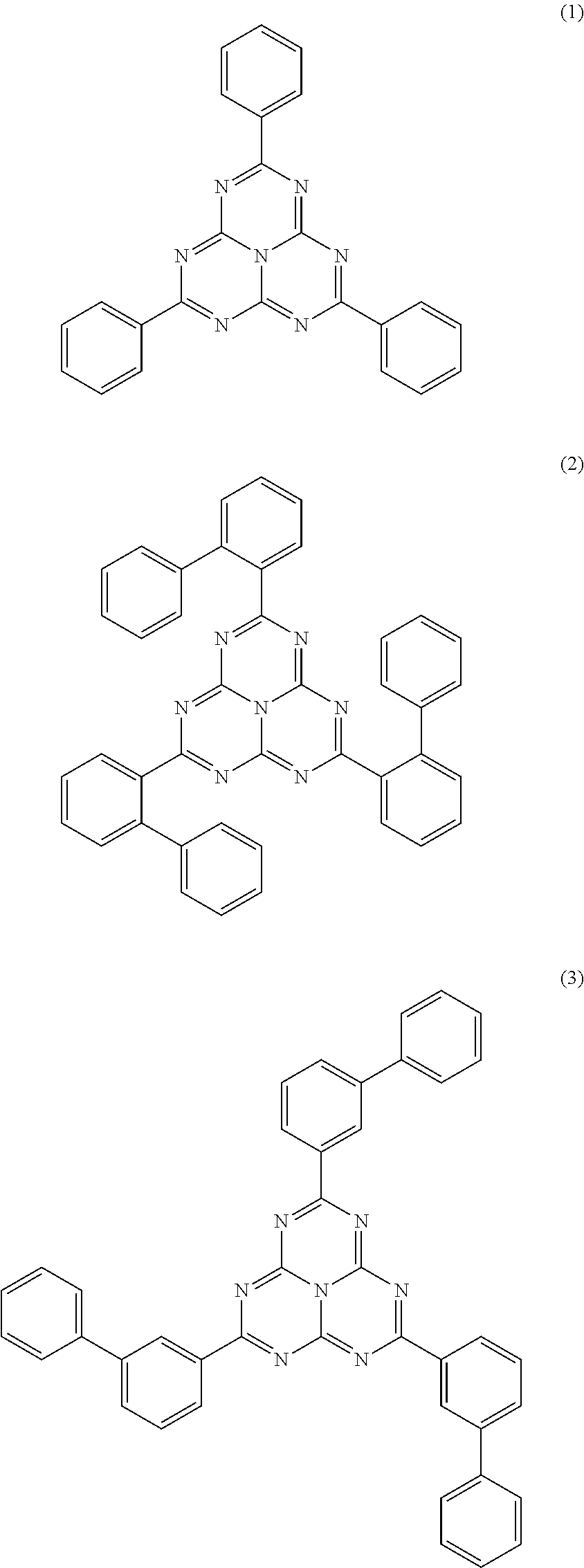
Organic electroluminescence devices containing substituted benzo[C]phenanthrenes
ActiveUS9006503B2Improve efficiencyImprove thermal stabilityOrganic chemistryElectroluminescent light sourcesArylBenzene
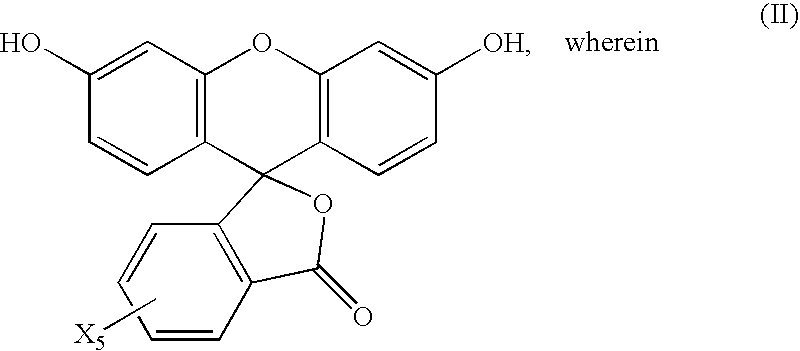
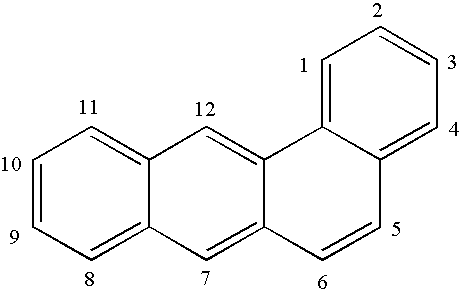
The present invention relates to substituted benzo[c]phenanthrene derivatives and to the production and to the use thereof in electronic devices, and to the electronic devices themselves. The present invention relates in particular an electronic device containing 10-benzo[c]phenanthrene derivatives of, e.g., formula (II)substituted with at least one aromatic unit or at least one diarylamino unit.
Owner:MERCK PATENT GMBH
Anthracene derivative and organic electroluminescence device using the anthracene derivative
ActiveCN105492413BImprove luminous efficiencyOrganic chemistrySolid-state devicesCarbon numberAnthracene
An anthracene derivative represented by the following formula (1). In formula (1), R 11 ~R 20 Either of the used with L 1 for bonding, not for use with L 1 The linkers are each independently a hydrogen atom, a halogen atom, a cyano group, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, and the like. L 1 It is a single bond, a substituted or unsubstituted divalent aromatic hydrocarbon group having 6 to 50 ring carbon atoms, and the like. Z is a structure represented by the following formula (2). In formula (2), R 1 , R 3 and R 4 Either of the used with L 1 for bonding, not for use with L 1 Bonder, R 2 , and R 5 ~R 10 Each independently represents a hydrogen atom, a halogen atom, a cyano group, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, and the like. Among them, R 5 ~R 8 At least one of the groups of two adjacent groups in the group is bonded to each other to form a saturated or unsaturated hydrocarbon ring.
Owner:IDEMITSU KOSAN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
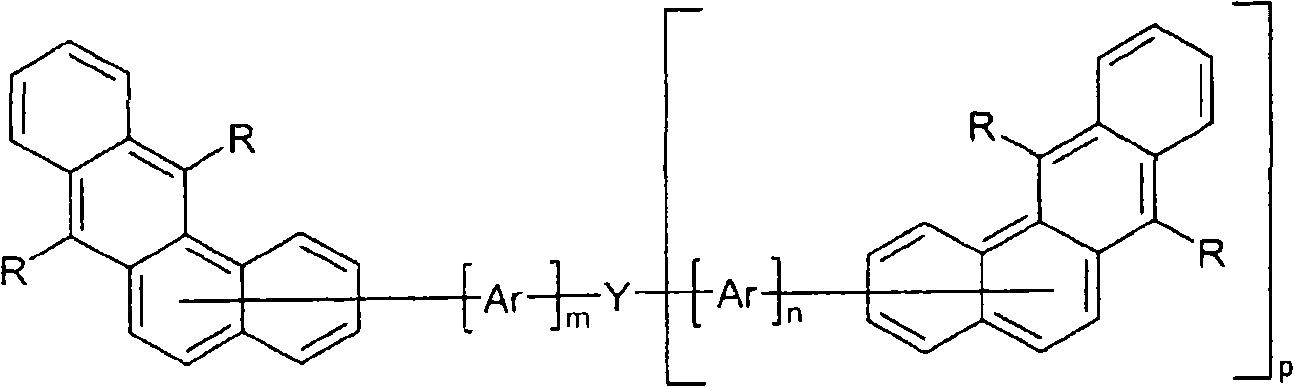
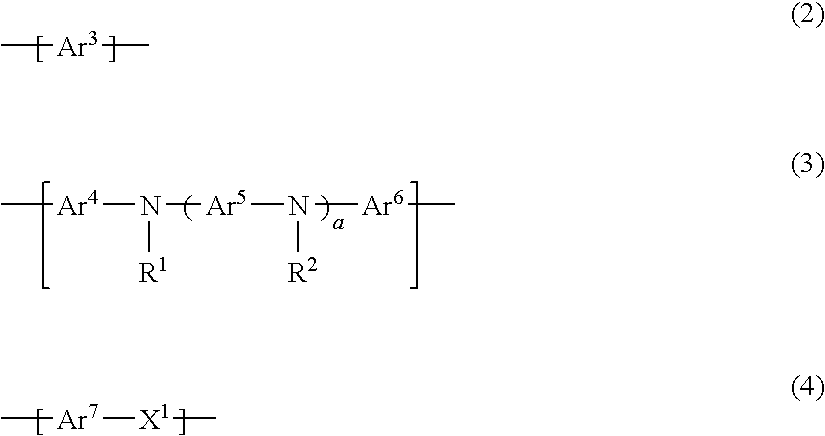
![Organic electroluminescence devices containing substituted benzo[C]phenanthrenes Organic electroluminescence devices containing substituted benzo[C]phenanthrenes](https://images-eureka.patsnap.com/patent_img/ce803416-f8ca-4f87-8b9e-ca8d04e59948/US09006503-20150414-C00001.PNG)
![Organic electroluminescence devices containing substituted benzo[C]phenanthrenes Organic electroluminescence devices containing substituted benzo[C]phenanthrenes](https://images-eureka.patsnap.com/patent_img/ce803416-f8ca-4f87-8b9e-ca8d04e59948/US09006503-20150414-C00002.PNG)
![Organic electroluminescence devices containing substituted benzo[C]phenanthrenes Organic electroluminescence devices containing substituted benzo[C]phenanthrenes](https://images-eureka.patsnap.com/patent_img/ce803416-f8ca-4f87-8b9e-ca8d04e59948/US09006503-20150414-C00003.PNG)