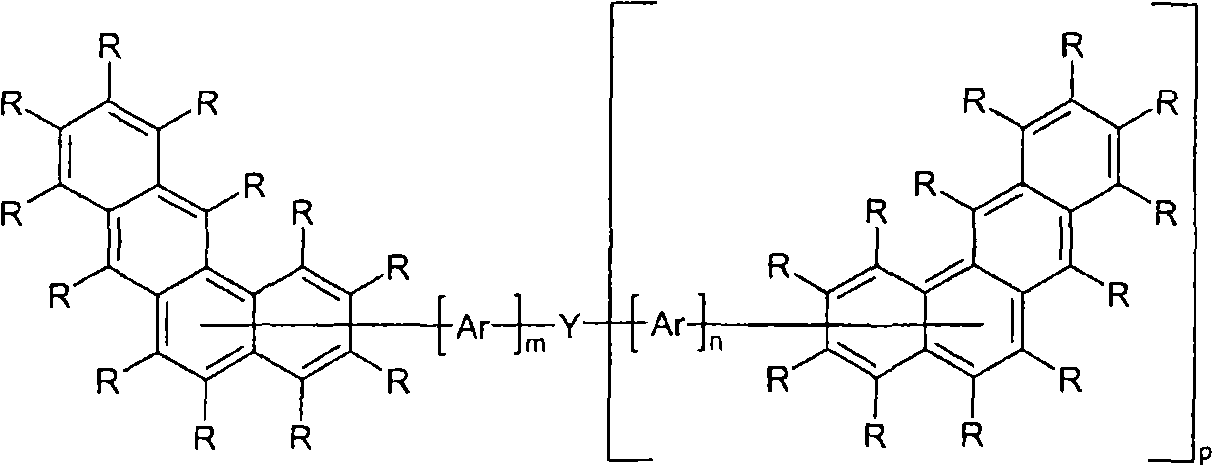
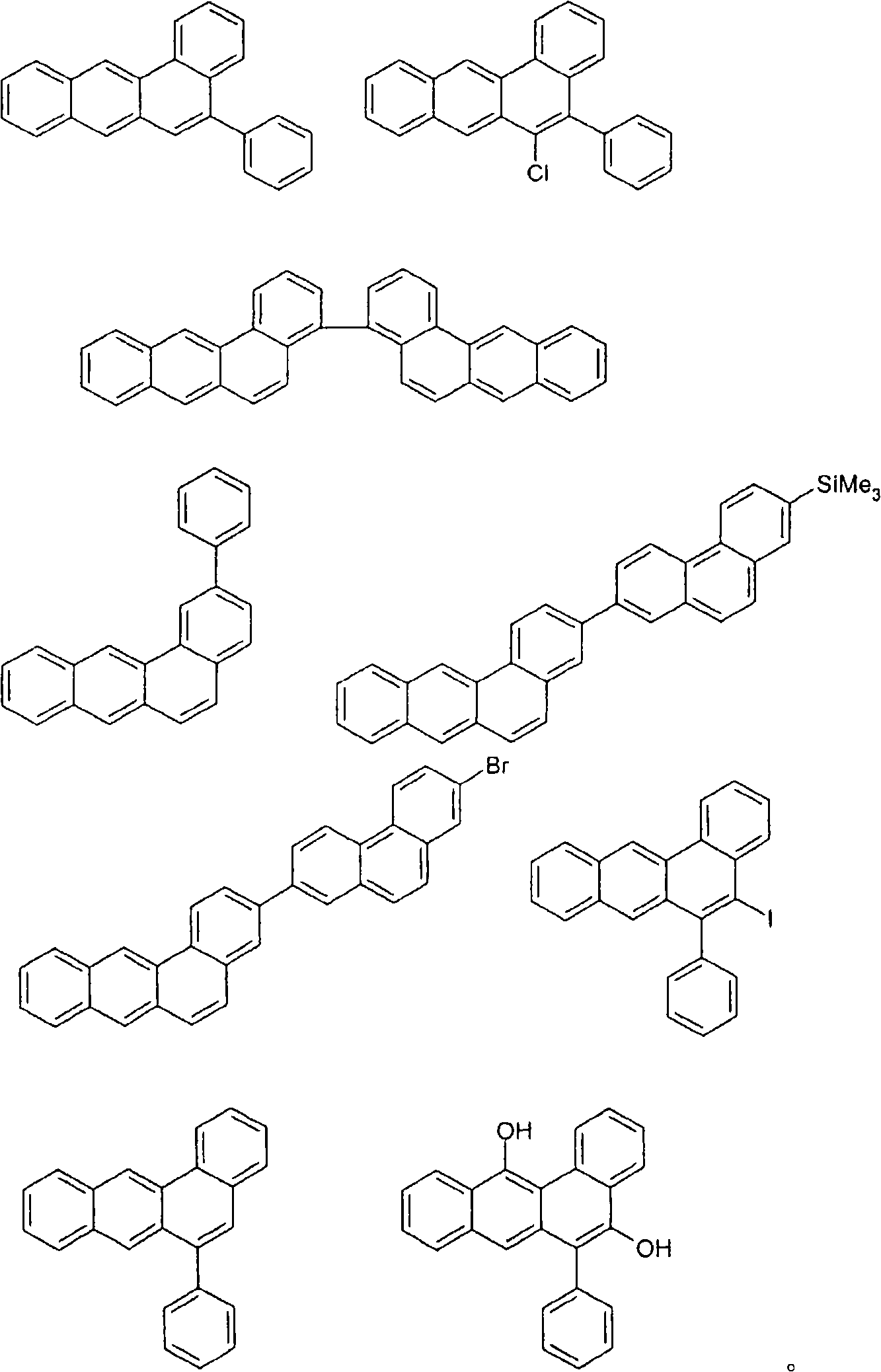
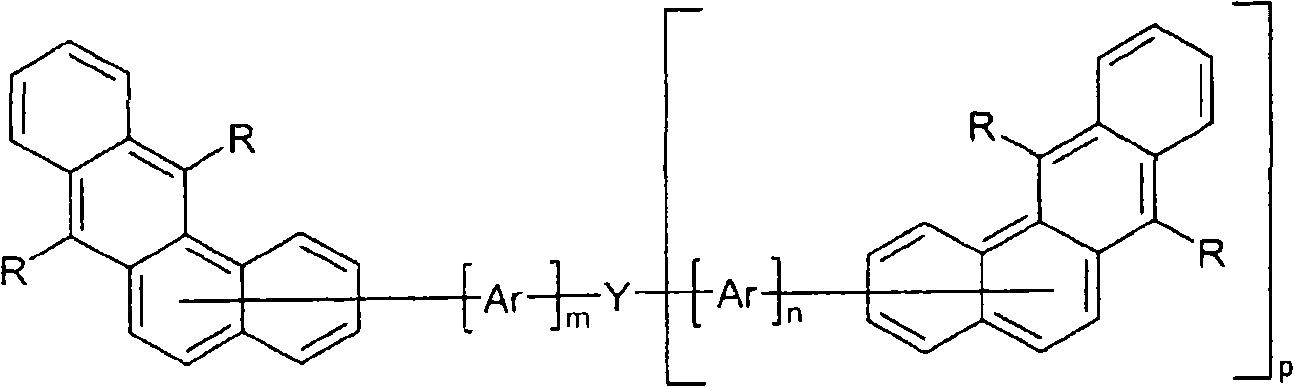Novel materials for organic electroluminescent devices
A technology of groups and atoms, applied in the direction of luminescent materials, electroluminescent light sources, electric solid-state devices, etc., can solve problems such as high technology, complexity, and thermal instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0171] For the preparation of oligomers or polymers, the monomers according to the invention are homopolymerized or copolymerized with other monomers. Suitable and preferred comonomers are selected from fluorene (eg according to EP 842208 or WO 00 / 22026), spirobifluorene (eg according to EP 707020, EP 894107 or WO06 / 061181), p-phenylene (eg according to WO 92 / 18552 ), carbazole (eg according to WO 04 / 070772 or WO 04 / 113468), thiophene (eg according to EP 1028136), dihydrophenanthrene (eg according to WO 05 / 014689 or WO 07 / 006383), cis and trans indeno Fluorenes (eg according to WO 04 / 041901 or WO 04 / 113412), ketones (eg according to WO 05 / 040302), phenanthrenes (eg according to WO 05 / 104264 or WO 07 / 017066) or multiples of these units. The polymers, oligomers and dendrimers usually also contain other units, for example light-emitting (fluorescent or phosphorescent) units, such as vinyltriarylamines (for example according to WO 07 / 068325) or phosphorescent metal complexes ( Fo...
Embodiment 1
[0196] Embodiment 1: the synthesis of 5-bromobenzo[a]anthracene
[0197] a) 2-(1-formylphenyl)-1-naphthylmethane
[0198]
[0199] The corresponding Grignard compound was prepared from 88.3 g (500 mmol) of 1-chloromethylnaphthalene and 12.2 g (500 mmol) of magnesium in 500 ml of ether. After the Grignard solution had been cooled to -78°C, 59ml (530mmol) of trimethyl borate was added, then the mixture was allowed to warm to room temperature, the solvent was removed in vacuo, 500ml of toluene, 92.5g (500mmol) of 2-Bromobenzaldehyde, 2.9 g (2.5 mmol) of tetrakis(triphenylphosphine)palladium(0) and 300 ml of 2M sodium carbonate solution are added to the residue and the reaction mixture is heated under reflux for 16 hours. After cooling, the organic phase is separated off, washed twice with 500 ml of water each, filtered through silica gel and evaporated to dryness, the residue is recrystallized from toluene / acetonitrile. Yield: 93.1 g (378 mmol), 75.6%, purity about 97% (NMR)...
Embodiment 2
[0206] Embodiment 2: the synthesis of benzo [a] anthracene-5-boronic acid
[0207]
[0208] 52ml (130mmol) of n-butyllithium (in n-hexane, 2.5M) was added dropwise at -78°C under vigorous stirring to 30.7g (100mmol) of 5-bromobenzo[a]anthracene in 1000ml of THF In the suspension, the mixture was stirred for another 2 hours. 16.7ml (150mmol) of trimethylborate was added to the red solution, stirred vigorously in one portion, the mixture was stirred at -78°C for an additional 30 minutes, then allowed to warm to room temperature over 3 hours, 300ml of water was added and the mixture was stirred for 30 minute. The organic phase was separated off and evaporated to dryness in vacuo. The solid was extracted in 100 ml of n-hexane, filtered with suction, washed once with 100 ml of hexane, and dried in vacuo. Yield: 24.8 g (91 mmol), 91 %, about 90 % pure (NMR) boronic acid with varying amounts of boric anhydride and boronic acid. Boronic acid can be used in this form without add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com