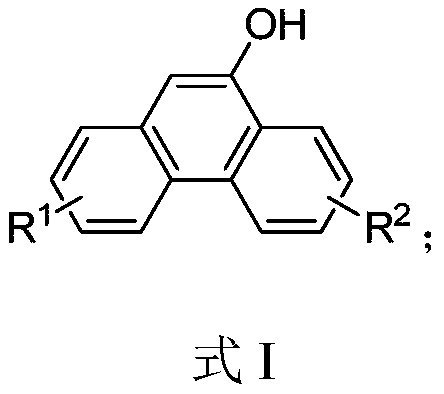
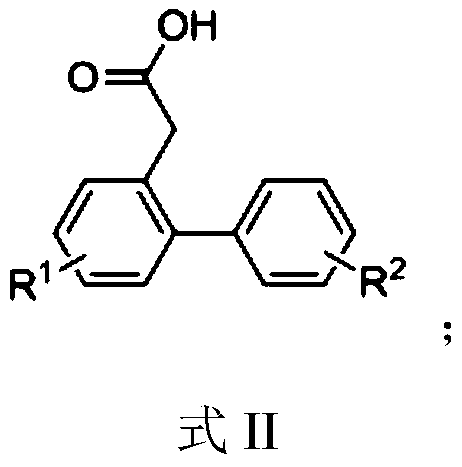
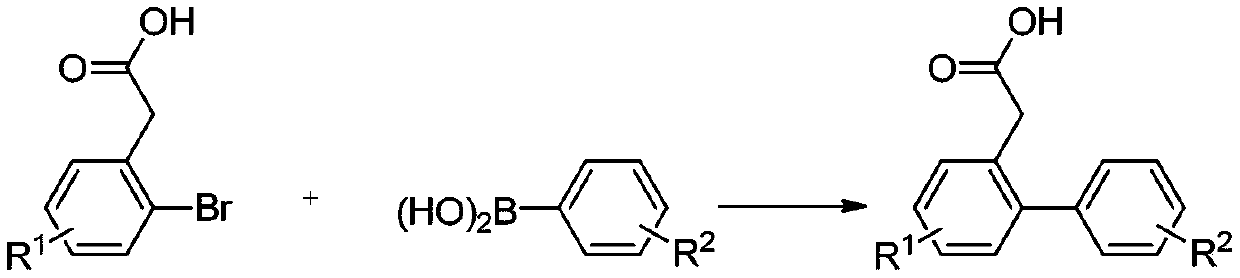
A kind of synthetic method of phenanthrene derivatives substituted by hydroxy
A synthesis method and derivative technology, applied in chemical instruments and methods, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of low yield and limited universality of substrates, etc. problem, to achieve the effect of short reaction time, cheap and easy-to-obtain catalyst, and good universality of functional groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of 2-carboxymethylbiphenyl
[0035]
[0036] Add 2-bromophenylacetic acid (430mg, 2mmol), phenylboronic acid (366mg, 3mmol), palladium acetate (1mol%, 4.5mg, 0.02mmol), sodium carbonate (636mg, 6mmol), DMF ( 2mL), water (2mL), and reacted in an oil bath at 100°C for 1h; after the reaction was completed, 10ml of saturated saline was added, and acidified to weak acidity with dilute hydrochloric acid; extracted with ethyl acetate (3×10mL), and desolvated under reduced pressure Afterwards, the target compound 2-carboxymethylbiphenyl was purified and separated by column chromatography as a white solid with a yield of 98%, m.p.114-115°C. 1 H NMR (400MHz, CDCl 3 )δ9.76(s,1H),7.43–7.24(m,9H),3.62(s,2H); 13 C NMR (101MHz, CDCl 3 )δ 178.39, 142.65, 140.94, 131.07, 130.42, 130.31, 129.25 (2C), 128.31 (2C), 127.63, 127.45, 127.30, 38.57.
[0037] According to the method of Example 1, different starting materials can be used to prepare different 2-carboxymethylbiphe...
Embodiment 2
[0091] Synthesis of 9-Hydroxyphenanthrene
[0092]
[0093] In a 10 mL round bottom flask, to a solution of 2-carboxymethylbiphenyl (106 mg, 0.5 mmol) and phosphorus pentoxide (213 mg, 1.5 mmol) in dichloromethane (2 mL) was added trifluoromethanesulfonic acid (375 mg , 2.5mmol), stirred at room temperature for 2h; after the reaction, the reaction solution was added dropwise to a mixed solution of water (10mL) and ethyl acetate (10mL) to quench; then extracted with ethyl acetate (3 × 10mL) , spin off the solvent, and purified by column chromatography to obtain the target compound 9-hydroxyphenanthrene as a white solid with a yield of 91%, m.p.150-151°C. 1 H NMR (400MHz, CDCl 3 )δ8.67(dd, J=8.4,0.8Hz,1H),8.58(dd,J=8.4,6.7Hz,1H),8.31(dd,J=8.0,1.2Hz,1H),7.79–7.60(m ,3H),7.60–7.45(m,2H),6.99(s,1H),5.21(s,1H). 13 C NMR (101MHz, CDCl 3 )δ149.50, 132.68, 131.58, 127.26, 126.98, 126.76 (2C), 126.45, 125.56, 124.32, 122.74, 122.61, 122.36, 106.14; HRMS (EI) calcd forC 14 h 10 ...
Embodiment 3
[0095] Synthesis of 9-Hydroxyphenanthrene
[0096] In a 10 mL round bottom flask, to 2-carboxymethylbiphenyl (106 mg, 0.5 mmol), phosphorus pentoxide (282 mg, 2 mmol), phenylacetic acid (102 mg, 0.75 mmol) was added methanesulfonic acid (2 mL) , reacted at room temperature for 2h; after the reaction, the reaction solution was added dropwise to a mixed solution of water (10mL) and ethyl acetate (10mL) to quench; then extracted with ethyl acetate (3×10mL), decompressed to Put the solvent in a 10mL round bottom flask, add sodium hydroxide (100mg, 25mmol), water (1mL) and ethanol (1mL), stir at room temperature for 2 hours, add saturated saline 10mL after the reaction, and extract with ethyl acetate (3×10mL), the solvent was spun off, and the synthesis of the target compound 9-hydroxyphenanthrene was obtained by purification and separation by column chromatography, the yield was 84%, and the characterization data were as in Example 2.
[0097] According to the method of Example 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com