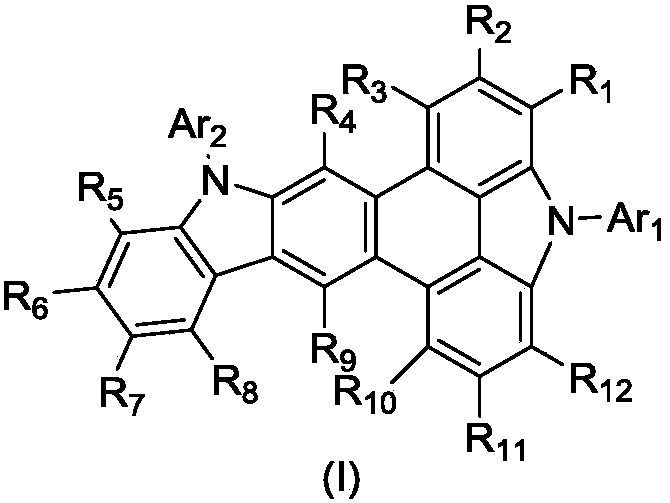
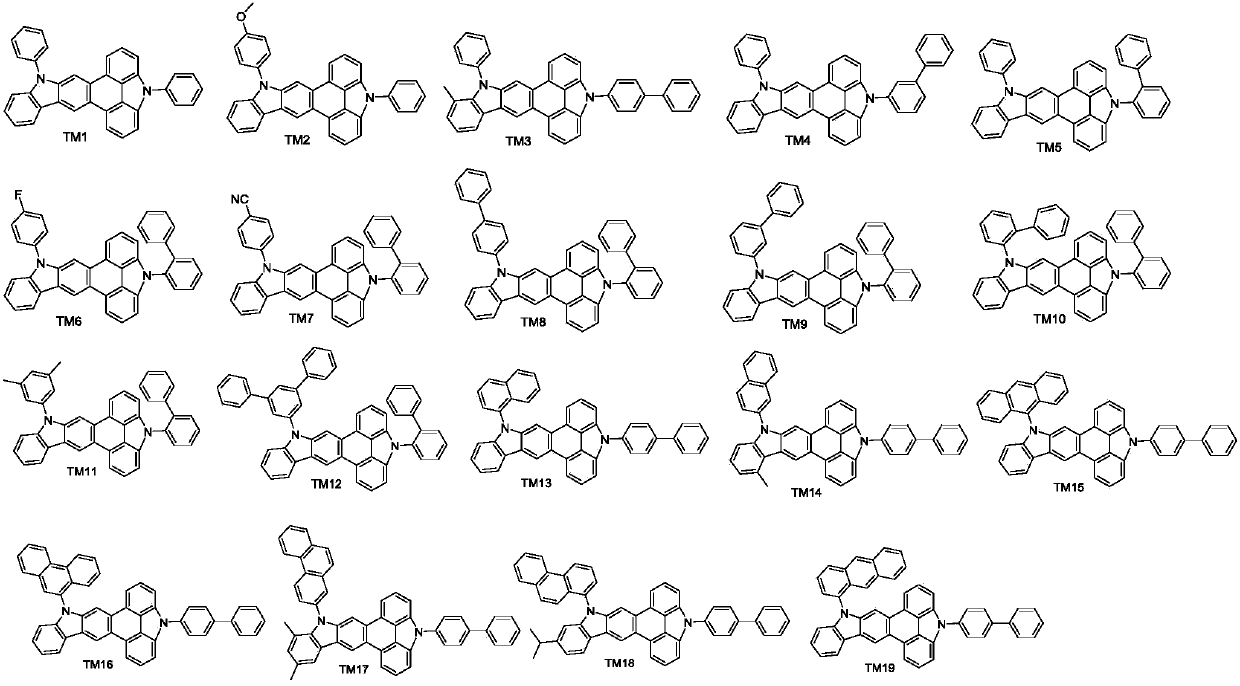
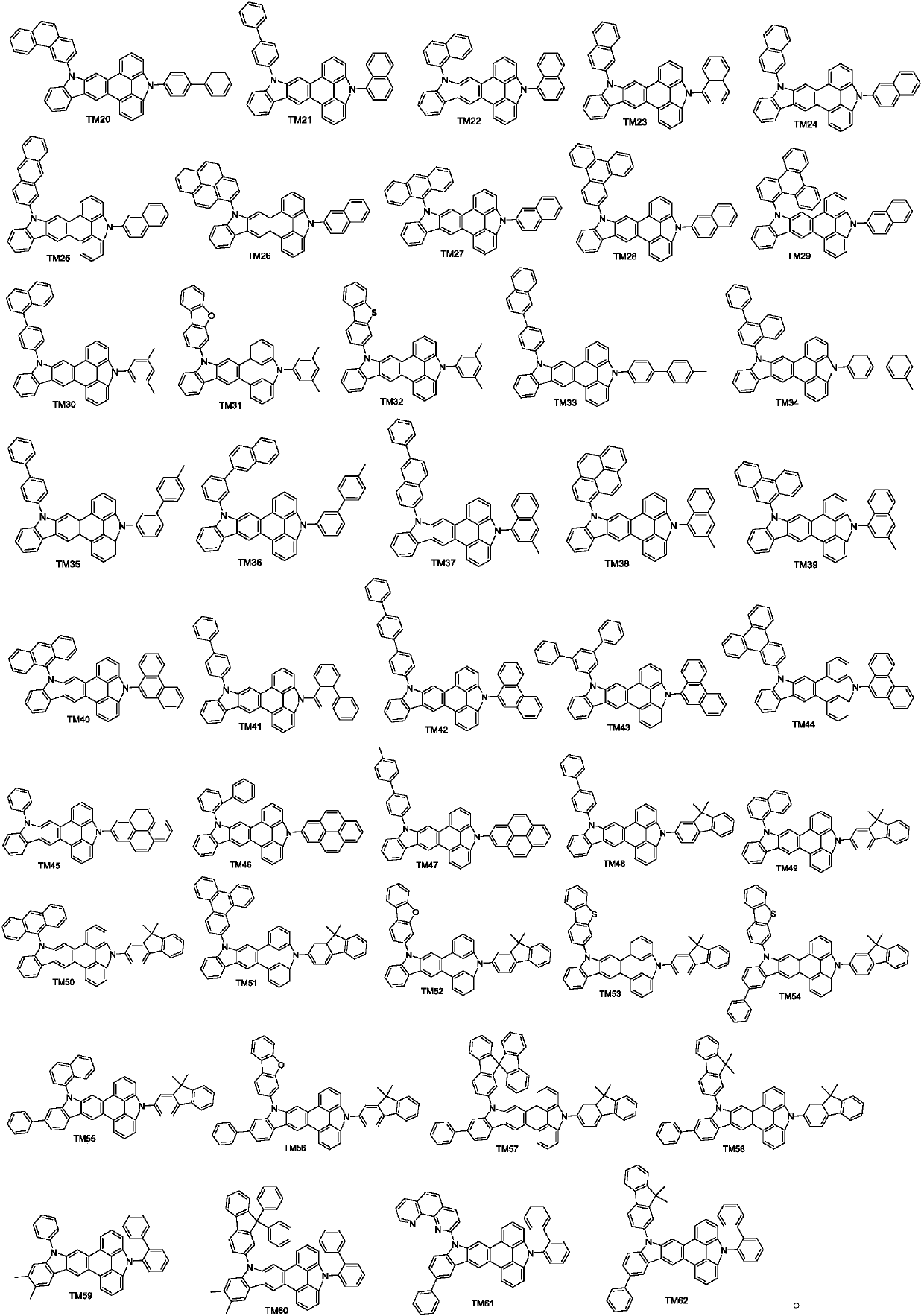
Triphenylene derivative and application thereof
A derivative, triphenylene technology, applied in the field of triphenylene derivatives, achieves the effects of easy-to-obtain raw materials, simple preparation methods, and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of intermediate A
[0039]
[0040] (1) Preparation of Intermediate A-1: Add 210ml of 2M potassium carbonate to 21.21g (105mmol) of 1-bromo-2-nitrobenzene and 42.86g (157.5mmol) of triphenylene-2-boronic acid Aqueous solution, 12.2g (10.5mmol) tetrakis(triphenylphosphine)palladium, 530ml toluene and 265ml ethanol were heated to 80°C and stirred for 12 hours. After cooling to room temperature, it was extracted with ethyl acetate, the organic layer was washed with distilled water, and then dried over anhydrous magnesium sulfate, and the organic solvent was distilled off under reduced pressure. The resulting residue was purified by column chromatography to obtain 31.18 g (89.25 mmol) of a solid, namely Intermediate A-1 (yield 85%).
[0041] (2) Preparation of intermediate A-2: replace 1-bromo-2-nitrobenzene with equimolar 1-bromo-3-methyl-2-nitrobenzene, other steps and intermediate A-1 The preparation is the same, and the intermediate ...
Embodiment 2
[0048] Embodiment 2: the preparation of intermediate B
[0049]
[0050] (1) Preparation of Intermediate B-1: Dissolve 34.75g (88mmol) of Intermediate A-1 in 440ml of o-dichlorobenzene, then add 58.2g (220mmol) of triethyl phosphite, heat to 150°C, and react 24 hours. Cooling, suction filtration, and distillation under reduced pressure to remove the solvent, and the resulting residue was purified by column chromatography to obtain 15.08 g (47.5 mmol) of intermediate B-1 with a yield of 54%.
[0051] (2) Preparation of Intermediate B-2: Intermediate A-1 is replaced by an equimolar amount of Intermediate A-2, and other steps are the same as the preparation of Intermediate B-1 to obtain Intermediate B-2 .
[0052] (3) Preparation of intermediate B-3: Intermediate A-1 is replaced by an equimolar amount of intermediate A-3, and other steps are the same as the preparation of intermediate B-1 to obtain intermediate B-3 . .
[0053] (4) Preparation of intermediate B-4: Interme...
Embodiment 3
[0058] Embodiment 3: the preparation of intermediate C
[0059]
[0060]
[0061] (1) Preparation of intermediate C-1: under argon atmosphere, 12.7g (40mmol) intermediate B-1 and 6.28g (40mmol) bromobenzene, 7.69g (80mmol) sodium tert-butoxide were dissolved in 200ml dry toluene 0.18g (0.8mmol) of palladium acetate and 0.16g (0.8mmol) of tri-tert-butylphosphine were added during stirring, heated to 80°C, and reacted for 8 hours. Cool, filter the reaction system through diatomaceous earth / silica gel, distill off the solvent from the filtrate under reduced pressure, recrystallize the resulting residue in toluene, and filter and dry the crude product to obtain 13.7g (34.8mmol) of intermediate C-1 , and the yield was 87%.
[0062] (2) Preparation of intermediate C-2: Bromobenzene was replaced by an equimolar amount of 4-bromoanisole, and other steps were the same as the preparation of intermediate C-1 to obtain intermediate C-2.
[0063] (3) Preparation of intermediate C-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com