Hydroxyl-substituted phenanthrene derivative synthesis method
A synthesis method and technology of derivatives, which are applied in chemical instruments and methods, preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of low yield and limited universality of substrates, etc. The problem is that the reaction time is short, the catalyst is cheap and easy to obtain, and the reaction yield is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
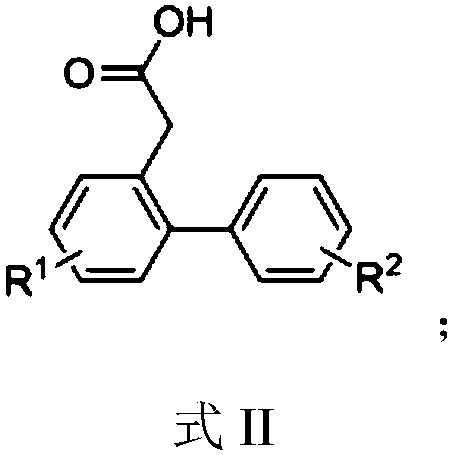
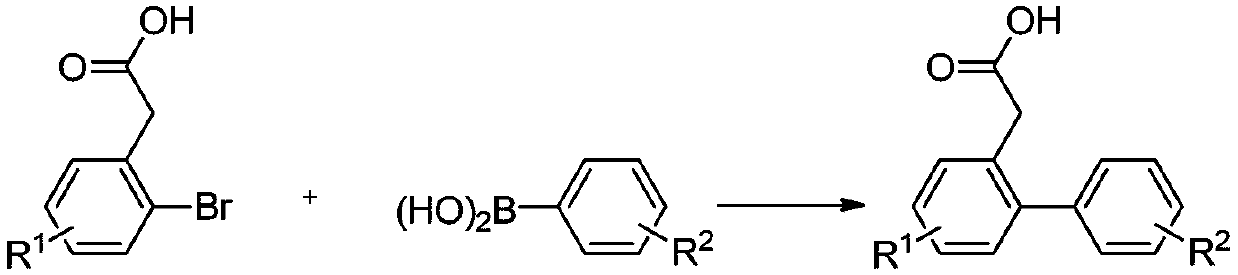
[0034] Synthesis of 2-carboxymethylbiphenyl
[0035]
[0036] Add 2-bromophenylacetic acid (430mg, 2mmol), phenylboronic acid (366mg, 3mmol), palladium acetate (1mol%, 4.5mg, 0.02mmol), sodium carbonate (636mg, 6mmol), DMF ( 2mL), water (2mL), and reacted in an oil bath at 100°C for 1h; after the reaction was completed, 10ml of saturated saline was added, and acidified to weak acidity with dilute hydrochloric acid; extracted with ethyl acetate (3×10mL), and desolvated under reduced pressure Afterwards, the target compound 2-carboxymethylbiphenyl was purified and separated by column chromatography as a white solid with a yield of 98%, m.p.114-115°C. 1 H NMR (400MHz, CDCl 3 )δ9.76(s,1H),7.43–7.24(m,9H),3.62(s,2H); 13 C NMR (101MHz, CDCl 3 )δ 178.39, 142.65, 140.94, 131.07, 130.42, 130.31, 129.25 (2C), 128.31 (2C), 127.63, 127.45, 127.30, 38.57.
[0037] According to the method of Example 1, different starting materials can be used to prepare different 2-carboxymethylbiphe...
Embodiment 2
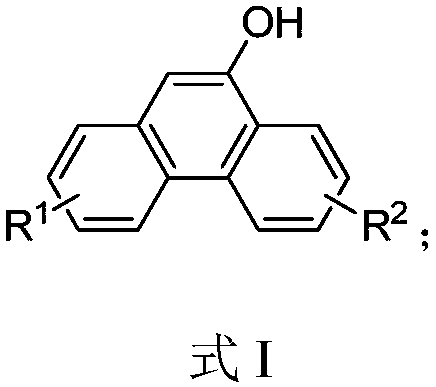
[0091] Synthesis of 9-Hydroxyphenanthrene
[0092]
[0093] In a 10 mL round bottom flask, to a solution of 2-carboxymethylbiphenyl (106 mg, 0.5 mmol) and phosphorus pentoxide (213 mg, 1.5 mmol) in dichloromethane (2 mL) was added trifluoromethanesulfonic acid (375 mg , 2.5mmol), stirred at room temperature for 2h; after the reaction, the reaction solution was added dropwise to a mixed solution of water (10mL) and ethyl acetate (10mL) to quench; then extracted with ethyl acetate (3 × 10mL) , spin off the solvent, and purified by column chromatography to obtain the target compound 9-hydroxyphenanthrene as a white solid with a yield of 91%, m.p.150-151°C. 1 H NMR (400MHz, CDCl 3 )δ8.67(dd, J=8.4,0.8Hz,1H),8.58(dd,J=8.4,6.7Hz,1H),8.31(dd,J=8.0,1.2Hz,1H),7.79–7.60(m ,3H),7.60–7.45(m,2H),6.99(s,1H),5.21(s,1H). 13 C NMR (101MHz, CDCl 3 )δ149.50, 132.68, 131.58, 127.26, 126.98, 126.76 (2C), 126.45, 125.56, 124.32, 122.74, 122.61, 122.36, 106.14; HRMS (EI) calcd forC 14 h 10 ...
Embodiment 3
[0095] Synthesis of 9-Hydroxyphenanthrene
[0096] In a 10 mL round bottom flask, to 2-carboxymethylbiphenyl (106 mg, 0.5 mmol), phosphorus pentoxide (282 mg, 2 mmol), phenylacetic acid (102 mg, 0.75 mmol) was added methanesulfonic acid (2 mL) , reacted at room temperature for 2h; after the reaction, the reaction solution was added dropwise to a mixed solution of water (10mL) and ethyl acetate (10mL) to quench; then extracted with ethyl acetate (3×10mL), decompressed to Put the solvent in a 10mL round bottom flask, add sodium hydroxide (100mg, 25mmol), water (1mL) and ethanol (1mL), stir at room temperature for 2 hours, add saturated saline 10mL after the reaction, and extract with ethyl acetate (3×10mL), the solvent was spun off, and the synthesis of the target compound 9-hydroxyphenanthrene was obtained by purification and separation by column chromatography, the yield was 84%, and the characterization data were as in Example 2.
[0097] According to the method of Example 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com