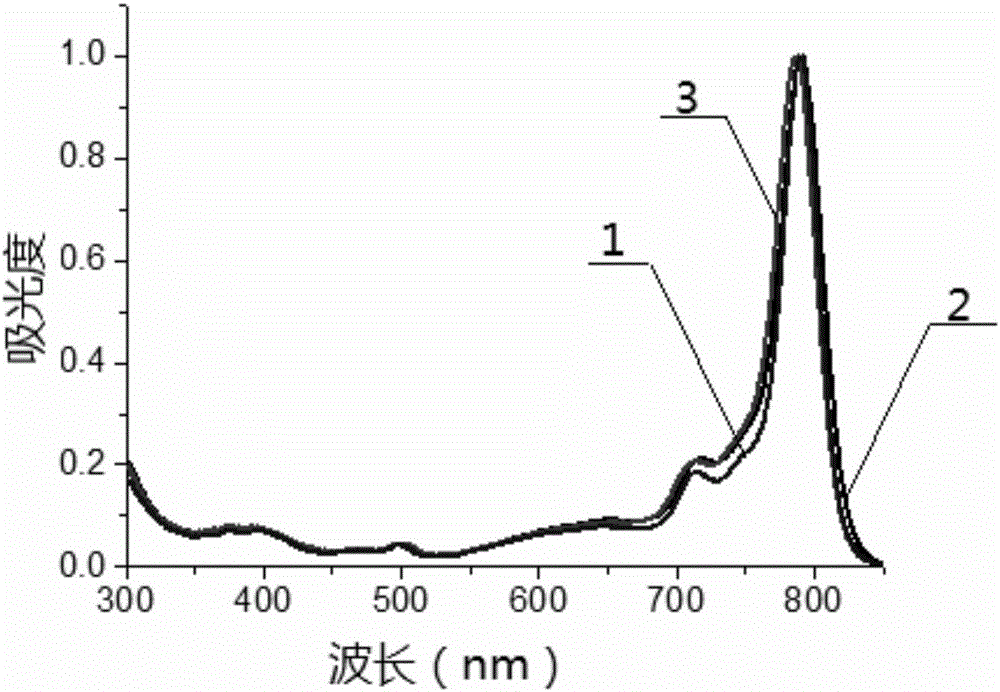
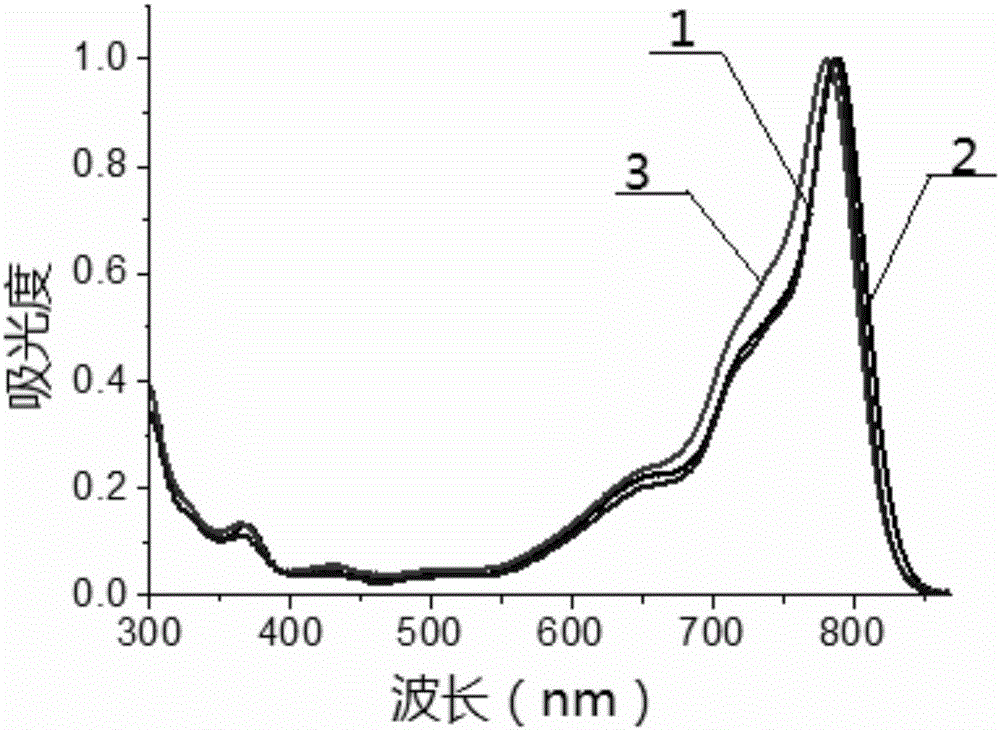
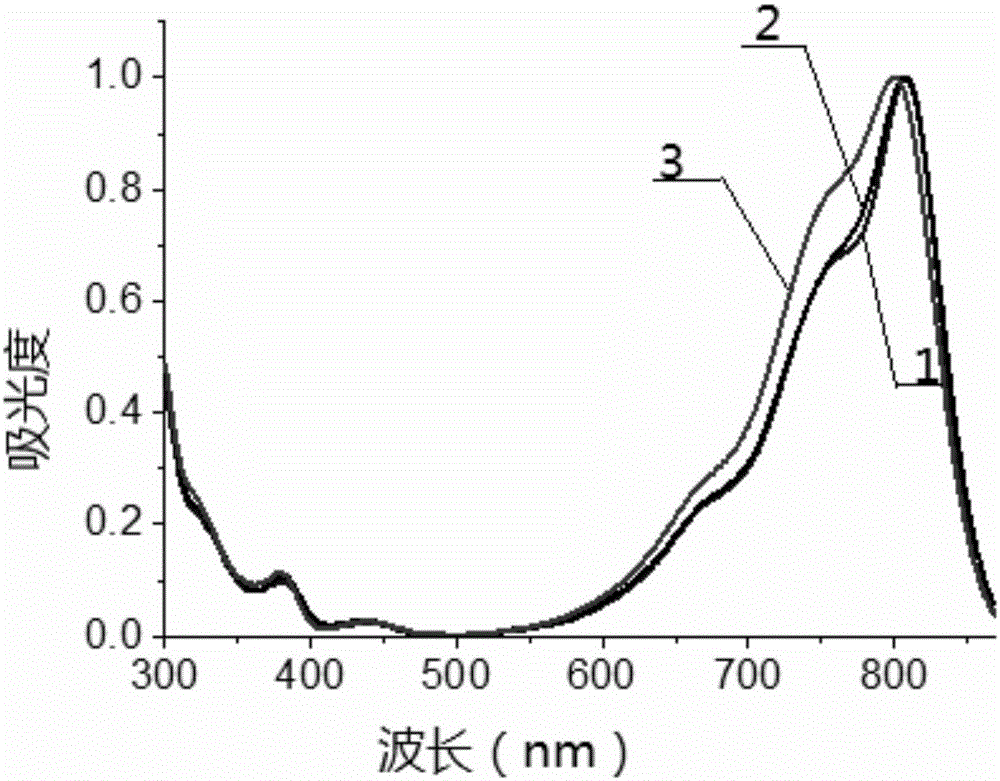
Beta-phenanthrene azaBODIPY dye and preparation method and application thereof
A technology of fluoroborate dipyrrole and phenanthrene nitrogen, which is applied in the field of β-phenanthreneazepine fluoroborate dipyrrole dye and its preparation, and can solve the problems of unsatisfactory absorption wavelength, few types, poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention also provides a method for preparing a β-phenanthrene azinafluoroboron dipyrrole dye with the structure shown in formula (I), wherein the preparation method comprises:
[0036] 1) In the presence of palladium, the compound represented by formula (II) is subjected to a first contact reaction with arylboronic acid to prepare a mixture M1;
[0037] 2) In the presence of triethylamine, the mixture M1 obtained in step 1) is subjected to a second contact reaction with boron trifluoride ether to obtain a mixture M2;
[0038] 3) The mixture M2 is subjected to a third contact reaction with an oxidizing agent to obtain a β-phenanthreneazepinefluoroboron dipyrrole dye with a structure shown in formula (I);
[0039]
[0040]
[0041] Among them, R 1 Is C1-C6 alkoxy; R 2 and R 4 Each independently selected from C1-C6 alkoxy or C1-C6 alkyl; R 3 and R 5 each independently selected from hydrogen or C1-C6 alkoxy; X is halogen.
[0042] Certainly, the mi...
preparation example 1
[0062] Put 9g of potassium hydroxide in 30g of methanol solution and mix to prepare a methanol solution of potassium hydroxide, add 8g of p-methoxyacetophenone and 8g of p-methoxybenzaldehyde into the above-mentioned methanol solution of potassium hydroxide and react for 30min , a large amount of precipitate was obtained, and the chalcone was obtained by suction filtration; 14 g of the chalcone prepared above was mixed with 6 g of nitromethane, and then 7 g of diethylamine and 50 g of methanol were added and mixed and refluxed for 12 hours to obtain a mixture M1 ; After distilling the solvent in the above-mentioned mixture M1 under reduced pressure, get the solid mixture M2; add 50 g of methanol and 15 g of ammonium acetate to the above-mentioned solid mixture M2 and reflux for 12 hours to obtain a red metallic luster powder M3; The obtained powder M3 was placed in a round-bottomed flask, and 100 g of toluene was added therein to dissolve it and heated to 60°C, then 10 g of tri...
preparation example 2
[0065] Prepare according to the preparation method of Preparation Example 1, except that p-methoxyacetophenone is replaced by p-tert-butylacetophenone, and p-methoxybenzaldehyde is replaced by 3,4,5-trimethoxy Benzaldehyde, compound A2 was obtained. The proton nuclear magnetic spectrum and carbon nuclear magnetic spectrum parameters of the obtained compound A2 are as follows: 1 HNMR (300MHz, CDCl3) δ: 8.07-8.03(m, 8H), 7.08-6.97(m, 8H), 6.92(s, 2H), 3.89(s, 6H), 3.88(m, 6H). 13 C NMR (75MHz, CDCl3) δ: 161.7, 160.7, 157.8, 145.1, 142.8, 131.5, 130.8, 125.5, 124.4, 117.1, 114.2, 55.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com