Coumarin-based fluorescent probe as well as preparation method and application thereof
A fluorescent probe, coumarin technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of background fluorescence interference, inapplicable cells, tissues and living imaging, etc., to avoid fluorescence quenching , avoid self-absorption effect, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
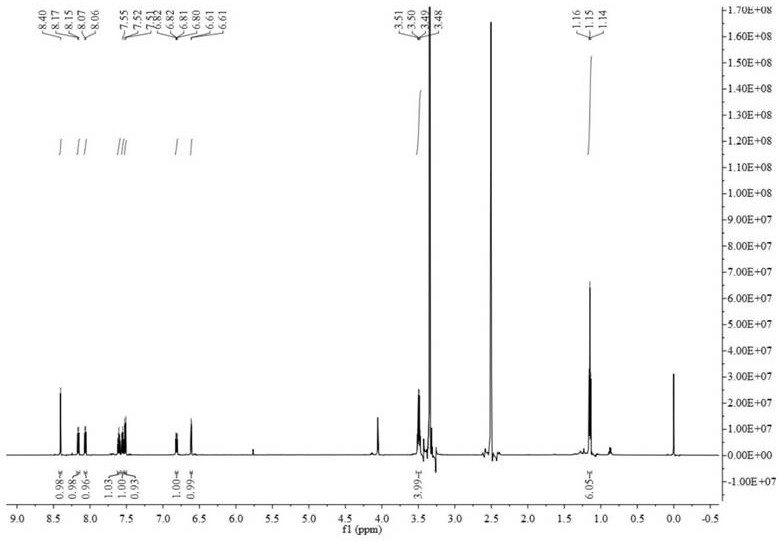
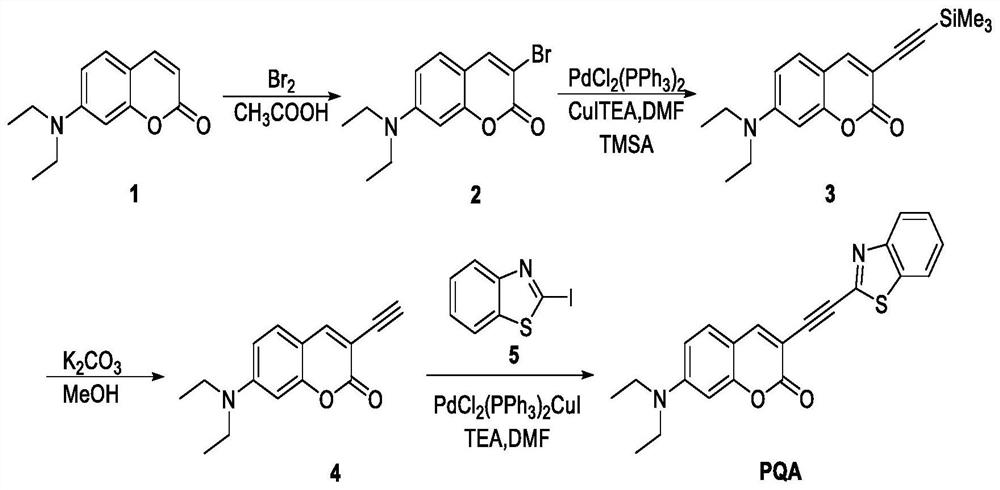
[0033] The preparation of fluorescent probe comprises the following steps ( figure 1 ):
[0034] (1) Synthesis of compound 2: Br 2 (0.26mL, 5.0mmol) was added dropwise to a solution of 7-diethylaminocoumarin (compound 1, 1.58g, 5.0mmol) in acetic acid (25mL), and stirred at room temperature for 1h. A white solid was obtained by filtration, washed three times with acetic acid, and dried in the air. Compound 2, 7-diethylamine-3-bromocoumarin, was obtained as a white solid after recrystallization from acetone (yield: 60%).
[0035] (2) Synthesis of compound 3: under nitrogen protection, PdCl 2 (PPh 3 ) 2 (91.2mg, 0.13mmol, 5.0mol%), CuI (24.8mg, 0.13mmol, 5.0mol%), triethylamine (770L, 5.5mmol), and trimethylsilylacetylene (469L, 5.0mmol) were added to Compound 2 (740mg, 2.5mmol) in anhydrous DMF (25mL) solution. The resulting mixture was reacted at 60°C for 5 hours. After the reaction solution was cooled, 150 mL of water was added and extracted with dichloromethane. The...
Embodiment 2
[0039] Spectral testing of fluorescent probe PQA
[0040] (1) Study on the ultraviolet absorption spectrum and fluorescence emission spectrum of probe PQA in different solvents.
[0041] Accurately weigh 3.7411mg compound PQA sample, dissolve it in a 10mL volumetric flask with dichloromethane solvent to prepare a concentration of 1.0×10 -3 mol / L mother solution, and then use a pipette gun to draw 100 μL of the mother solution into a 10mL volumetric flask. -5 mol / L of the solution to be tested. The ultraviolet absorption spectrum and fluorescence emission spectrum of the probe PQA in different solvents were measured with the solution to be tested.
[0042] Table 1 The data of the ultraviolet absorption and fluorescence emission spectra of PQA in different solvents
[0043]
[0044] from Figure 4 It is concluded that the maximum absorption spectrum of the probe PQA has a strong red shift with the increase of the solution polarity. For example, among the above solvents, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com