Process for producing fluorescent reagent
A fluorescent reagent and solvent technology, applied in the field of preparation of fluorescent reagents, can solve the problems of high fluorescence quantum efficiency detection of components, unstable derivative products, low reactivity, etc., and achieve good application prospects, stable derivative products, and derivatization speed. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
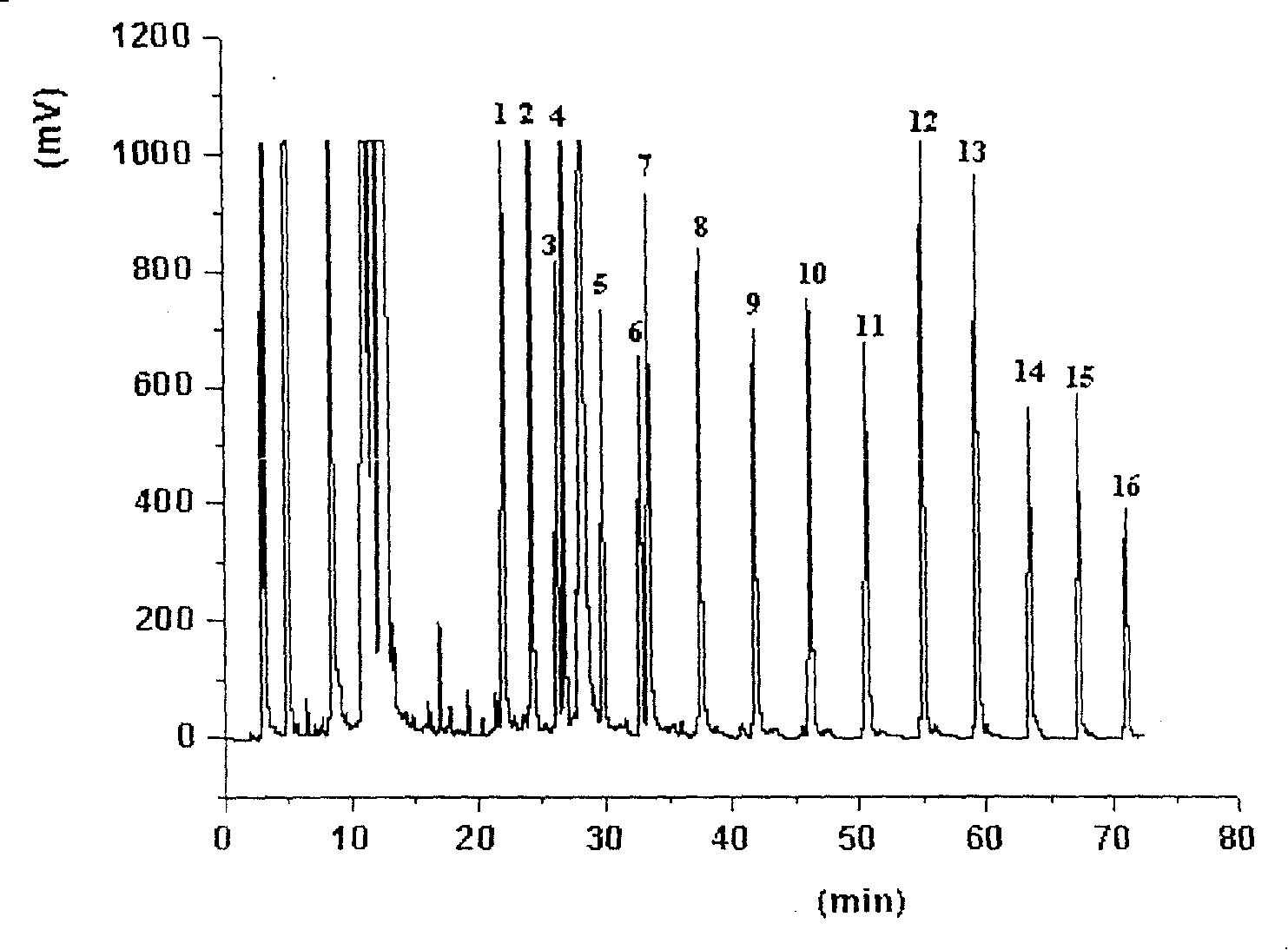
Image
Examples
Embodiment Construction
[0014] Add 46g of aniline o-benzoate and 100mL of concentrated sulfuric acid into a 500mL flask, heat in a boiling water bath for 4 hours, then pour it into a 1-liter beaker, pay attention to let the reaction solution flow down the wall of the vessel to reduce splashing of the solution. After boiling for 5 minutes, the yellow precipitate was filtered out, and the filtered solid was fully washed with water, and the crude acridone was air-dried.
[0015] Add 30g of the above-mentioned acridone and 150mL of dimethyl sulfoxide into a 500ml round-bottomed flask, start electromagnetic stirring and heat, add 10g of potassium hydroxide in batches within 20 minutes, keep the solution temperature at 100°C and heat under reflux and stir for 30 minutes , 40g of 2-bromoethanol was added within 1 hour, and the temperature continued to be kept at 100° C., and the reaction was continued under reflux and stirring for 2.5 hours, and the solution gradually turned dark brown. Cool the mixture in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com