Compound, preparation method and application of compound as fluorescent probe
A compound and mixture technology, applied in the field of fluorescent probes, can solve the problems of limited penetration depth of organisms, inability to realize PGP-1 detection and imaging, high detection limit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
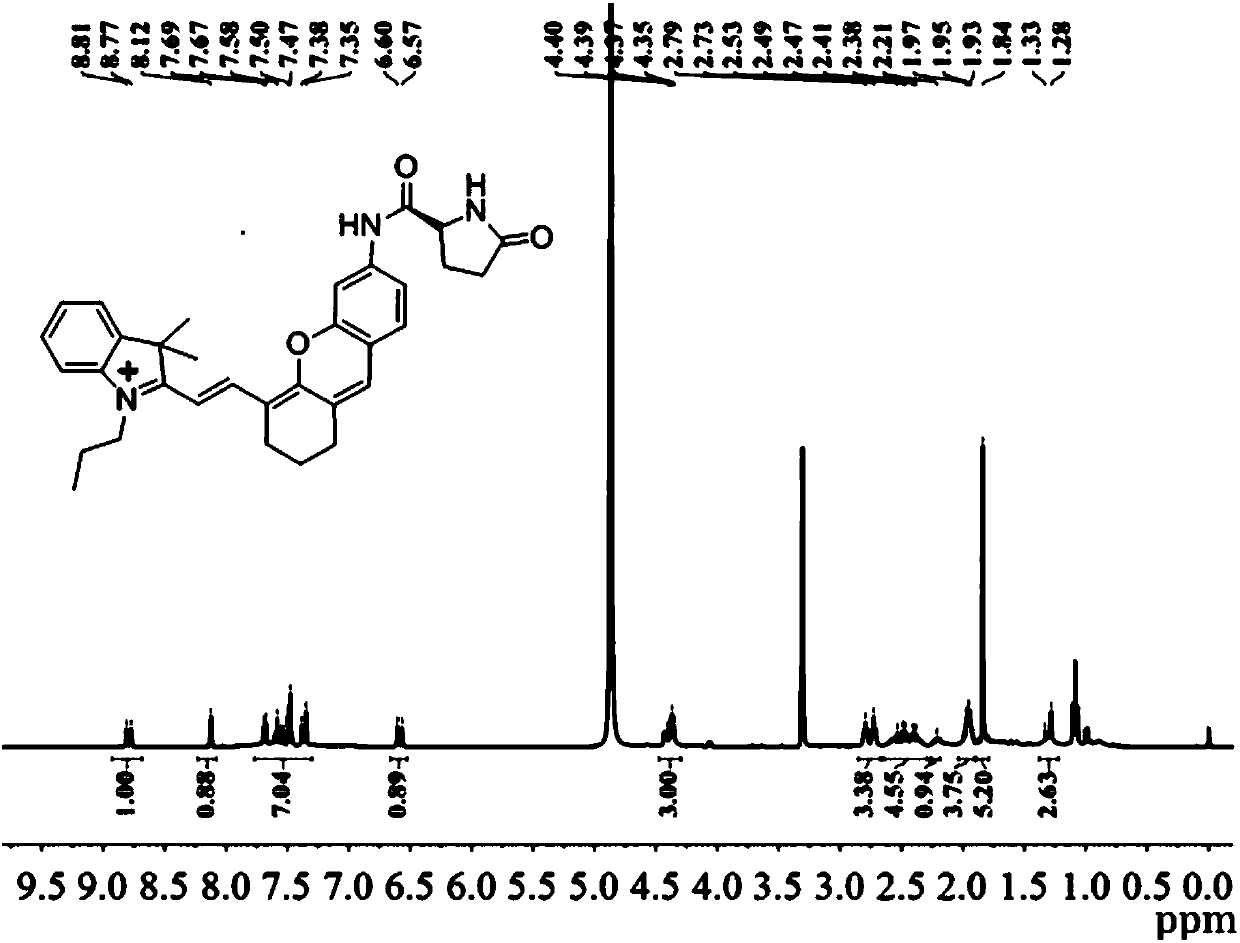
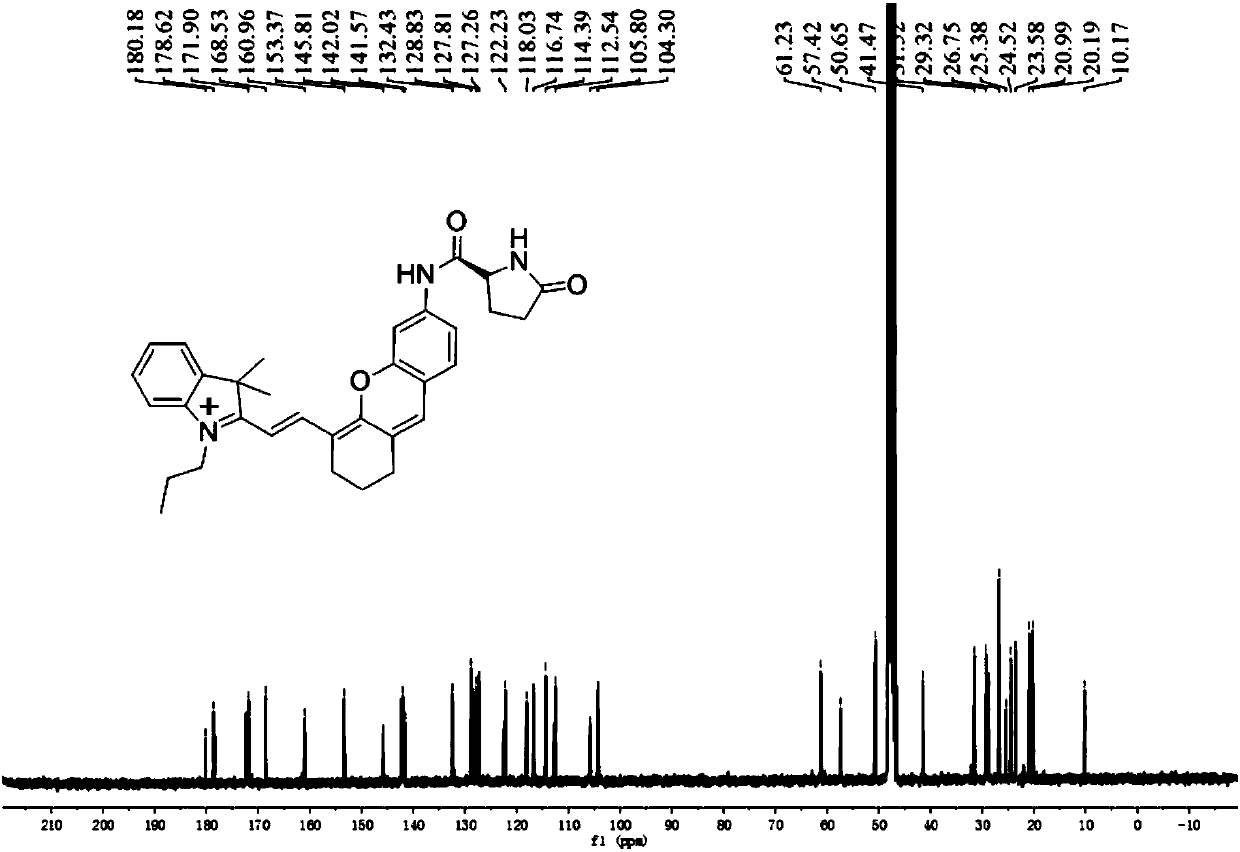
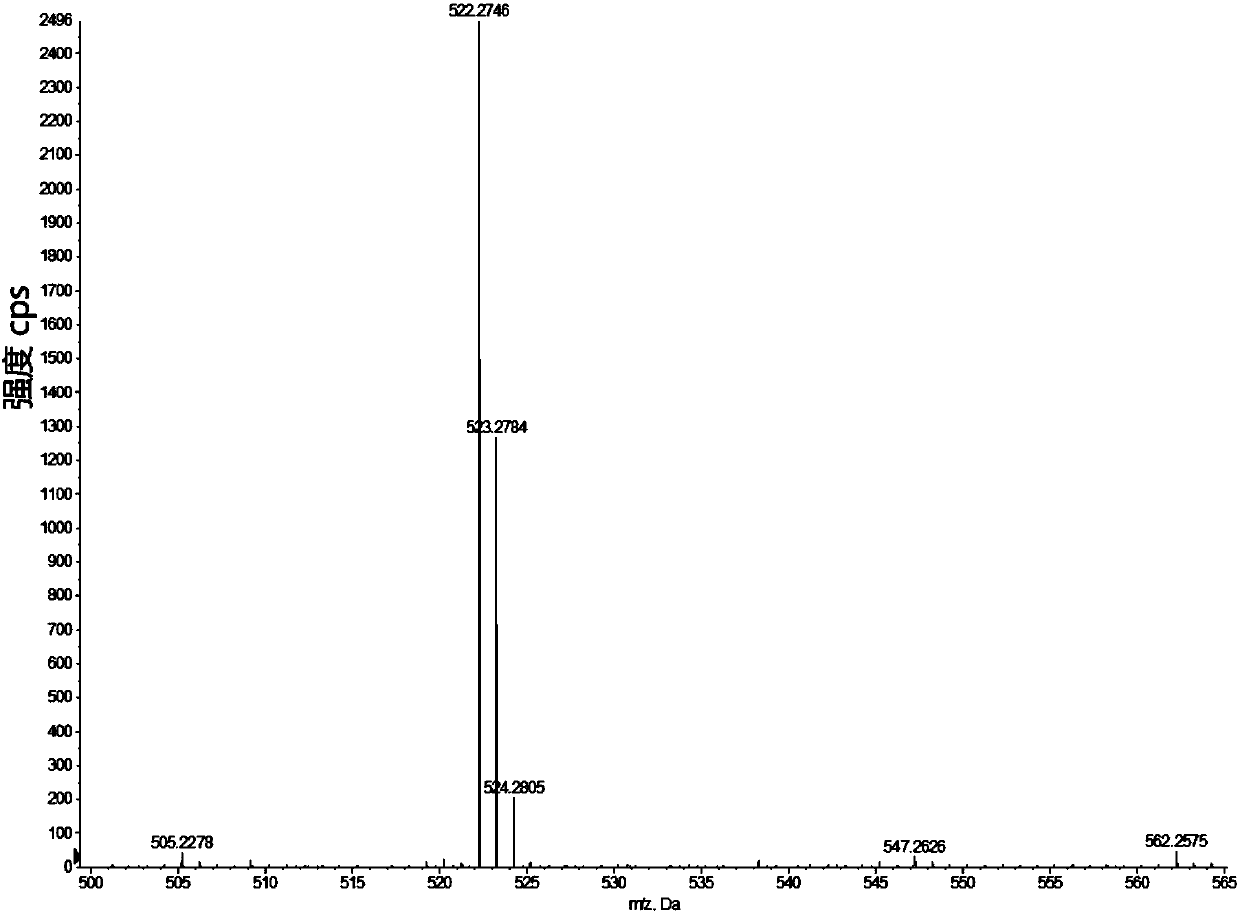
[0096] Example 1 Compound I Sample 1 # preparation of
[0097] In a 100ml round bottom flask, add IR-780 iodine compound (1mmol) and m-nitrophenol (1mmol) dissolved in 10mL of organic solvent acetonitrile, add 2mol of alkaline activator Na 2 CO 3 , stirred at room temperature for 4 hours, washed three times with water, and dried by rotary evaporation at 40°C to obtain mixture A. Add 2 mol of catalyst SnCl to mixture A 2 , under the protection of nitrogen, reacted at 70°C for 8 hours, washed the mixture three times with water, and dried by rotary evaporation at 40°C to obtain mixture B. BOC-pyroglutamic acid was activated by DIPEA and HATU to obtain activated BOC-pyroglutamic acid. 2 mol of activated BOC-pyroglutamic acid was added to mixture B, reacted at room temperature for 8 hours, washed with water three times, and dried by rotary evaporation at 40°C to obtain mixture C. Slowly add 2ml of trifluoroacetic acid dropwise to mixture C at 0°C, react at room temperature for...
Embodiment 2
[0099] Example 2 Compound I Sample 2 # ~Sample 6 # preparation of
[0100] The specific steps are the same as sample 1 # The difference is that the preparation conditions are changed according to table 1 (unspecified conditions in table 1 are the same as sample 1 in embodiment 1 # The same preparation conditions), to obtain sample 2 # ~Sample 6 # .
[0101] Table 1
[0102]
[0103] sample 2 # ~Sample 6 # NMR characterization results and high-resolution mass spectrometry of the same sample 1 # .
Embodiment 3
[0104] Example 3 Compound I Sample 7 # preparation of
[0105] (1) In a 100 ml round bottom flask, add 2,3,3-trimethylindole, 4-bromobutyric acid and 1,2 dichlorobenzene, heat up to 110 degrees Celsius and react for 12 hours, ethyl acetate precipitates, Suction filtration to obtain a pink precipitate; (2) Add dichloromethane, N,N-dimethylformamide, phosphorus oxychloride to cyclohexanone, react under reflux for 2 hours, pour into water for precipitation, Suction filtration to obtain a yellow precipitate; (3) The precipitate obtained in (1) and (2) was added to the acetic anhydride solvent, and sodium acetate was added at 70 degrees Celsius for 40 minutes and then poured into the aqueous solution for precipitation to obtain a green (4) Add m-nitrophenol and an appropriate amount of catalyst to (3), under nitrogen protection, react overnight at 70 degrees Celsius, wash the mixture three times, and spin dry the solvent; Add amino acid to (4), react at room temperature for 8 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com