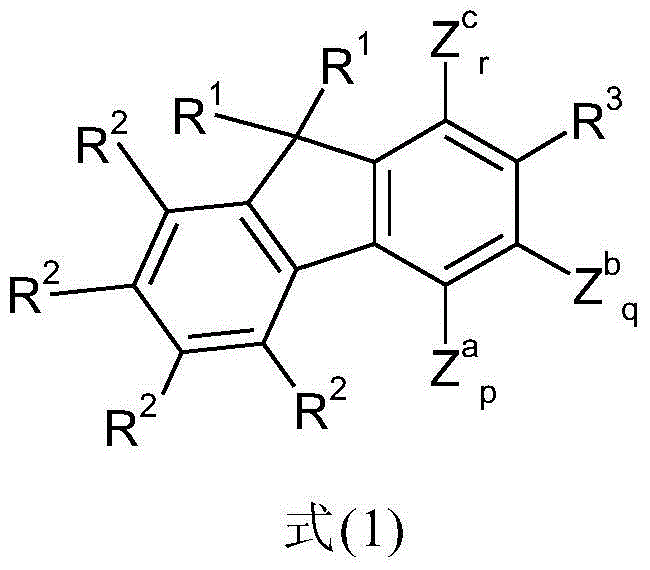
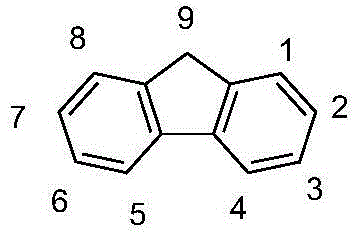
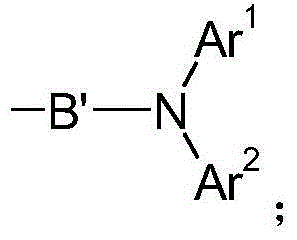Fluorenes and electronic devices containing them
A technology of atoms and groups, applied in the field of new organic compounds, can solve problems such as poor performance data and high working voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0278] Synthesis of compound biphenyl-4-yl-(9,9-diphenyl-9H-fluoren-4-yl)amine (1-1) and compound (1-2) to (1-12)
[0279]
[0280] 4-bromo-9,9-diphenyl-9H-fluorene
[0281] 37 g (152 mmol) of 2,2'-dibromobiphenyl was dissolved in 300 ml of dry THF in a flask that had been dried by heating. The reaction mixture was cooled to -78°C. At this temperature, 75 ml of a 15% solution of n-BuLi (119 mmol) in hexane was slowly added dropwise (duration: about 1 hour). The batch was stirred for another hour at -70°C. Subsequently 21.8 g of benzophenone (119 mmol) was dissolved in 100 ml of THF and added dropwise at -70°C. When the addition is complete, slowly warm the reaction mixture to room temperature and use NH 4 Cl is quenched and then evaporated in a rotary evaporator. 510 ml of acetic acid was carefully added to the evaporated solution, and then 100 ml of fuming hydrochloric acid was added. The batch was heated to 75°C and held at this temperature for 4 hours. During this time a w...
Embodiment 2
[0293] Compound biphenyl-3-ylbiphenyl-4-yl-(9,9-dimethyl-9H-fluoren-3-yl)amine (2-1) and compounds (2-2) to (2-10) Synthesis
[0294]
[0295] 3-bromo-9,9-dimethyl-9H-fluorene
[0296] 29.5 g (120 mmol) of 3-bromo-9H-fluorene (Tetrahedron Letters, 51, 37, 4894-4897; 2010) was dissolved in 220 ml of dry DMSO in a flask that had been dried by heating. Add 34.7g (361mmol) NaO at room temperature t Bu. The suspension was brought to an internal temperature of 65°C. At this temperature, a solution of 22.5 ml (361 mmol) of methyl iodide in DMSO (50 ml) was added dropwise at a rate such that the internal temperature did not exceed 65° C. (duration: about 30 minutes). Keep the batch at an internal temperature of 65°C for another 30 minutes, then pour 400ml of ice-cold NH 4 OH aqueous solution (1 / 1, v / v) and stir for about 20 minutes. Suction to filter out the precipitated solid and use about 200ml H 2 O and methanol are washed sequentially. Yield: 31 g (114 mmol) (95% of theory).
[029...
Embodiment 3
[0305] Compound biphenyl-2-ylbiphenyl-4-yl-(9,9-diphenyl-9H-fluoren-3-yl)amine (3-1) and compounds (3-2) to (3-5) Synthesis
[0306]
[0307] 3-bromo-9,9-diphenyl-9H-fluorene
[0308] 50 g (193 mmol) of 3-bromo-9H-fluorenone (Tetrahedron (tetrahedron), 51, 7, 2039-54; 1995) was dissolved in 500 ml of dry THF in a flask dried by heating. The clear solution was cooled to -10°C and then 70.7 ml (212 mmol) of 3M phenyl magnesium bromide solution was added. The reaction mixture was slowly warmed to room temperature, and then NH 4 Cl (500ml) quenched. The mixture was then partitioned between ethyl acetate and water, and the organic phase was washed three times with water, 2 SO 4 Dry and evaporate in a rotary evaporator. The crude product was recrystallized from heptane / toluene. 400 ml of benzene was added to the residue. The batch was heated to 50°C, and then 18.6 ml of trifluoromethanesulfonic acid was added dropwise. After 30 minutes, the reaction mixture was cooled to room tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com