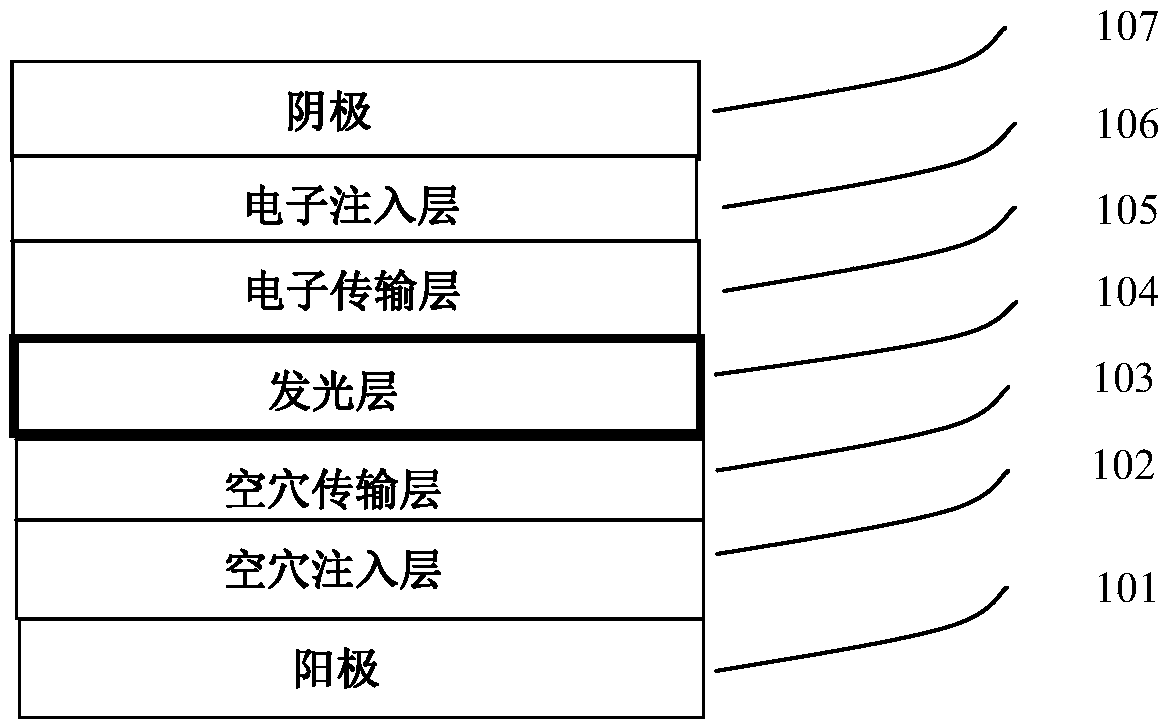
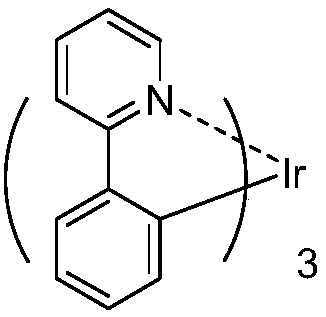
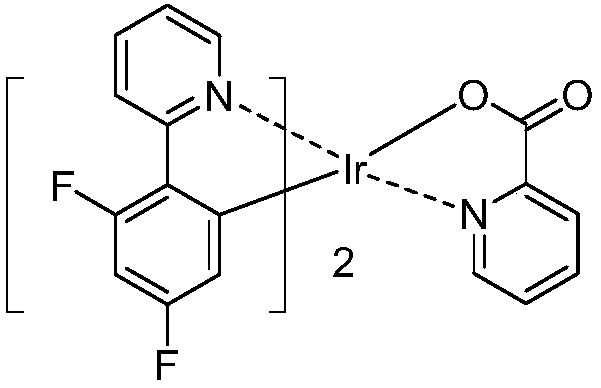
Organic metal iridium compound and organic light-emitting device (OLED)
An organic light-emitting device and organometallic technology, applied in indium organic compounds, platinum group organic compounds, organic chemistry, etc., can solve the problems of material melting point and heat resistance decline, unfavorable display materials, etc., to improve photoelectric performance and reduce stacking Function, the effect of lowering the sublimation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of organometallic iridium compound R-2:
[0023]
[0024] 1.1 Synthesis of TM-1:
[0025] Under nitrogen protection conditions, quinoline-4-carboxylic acid 17.3g (100mmol) was dissolved in 100mL tetrahydrofuran, mixed with 157mL (220mmoL) sec-butyllithium (1.4M) at -40°C, removed from the freezer, and the reaction solution Place in the water tank at room temperature for 60 minutes, stir for 2 hours, cool at -78°C, add dropwise 100mL of tetrahydrofuran mixed with 14.4g (150mmol) methanesulfonic acid, remove the cold water tank, and place the mixture in the water tank at room temperature for 30 minutes, then cool at 60°C Reflux for 5 hours.
[0026] After the reaction stopped, it was washed with saturated sodium chloride, and then 2N aqueous HCl was added, stirred for 30 minutes, and then extracted with ether. Use anhydrous magnesium sulfate to remove the moisture in the organic layer, filter with suction, and concentrate the obtained comp...
Embodiment 2
[0045] Embodiment 2: the synthesis of organometallic iridium compound R-31:
[0046]
[0047] 1.1 Synthesis of TM-1:
[0048] 1-Bromo-2-(2-phenylpropane)benzene (21.5 g, 78.5 mmol) in THF (500 ml) was treated with 31.8 mL of n-BuLi (2.5 M in hexane, 78.5 mmol) at -78 °C under nitrogen. solution in. The mixture was stirred for 30 minutes. A solution of quinolin-4-one (12.16 g, 78.5 mmol) in 300 mL THF was added dropwise. The reaction was allowed to proceed at -78°C for 30 minutes, then stirred at room temperature overnight. The reaction was quenched with water, and the solid was filtered. A mixture of the alcohol, acetic acid (220 mL) and concentrated HCl (10 mL) was refluxed for 2 hours without further purification.
[0049] After cooling, the mixture was filtered and washed with water, and dried under vacuum. The product was isolated in the form of a white solid (24 g, 92% of theory).
[0050] 1.2 Synthesis of TM-2:
[0051] Under the condition of nitrogen protecti...
Embodiment 3
[0062] Embodiment 3: the synthesis of organometallic iridium compound R-84:
[0063]
[0064] 1.1 Synthesis of TM-2:
[0065] Under the condition of nitrogen protection, TM-1 15.5g (100mmol) was dissolved in 500mL dry dichloromethane, 52g m-CPBA (300mmoL) was added at room temperature, and the reaction was carried out at room temperature for 16h.
[0066] After the reaction stopped, 500 mL of ice water was added to the reaction system, and after stirring for 30 minutes, precipitation occurred. Suction filtration under reduced pressure, washing with water for several times, recrystallization from ethanol to obtain 17.1 g (90%) of intermediate TM-2, HPLC: 99.0%.
[0067] 1.2 Synthesis of TM-3:
[0068] Under the condition of nitrogen protection, TM-2 15.2g (80mmol) was dissolved in 500mL dry THF, 45g 2-tributylstannylthiophene (120mmoL) was added at room temperature, nitrogen was replaced three times, and the temperature was raised to 70°C for 16h .
[0069] After the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com