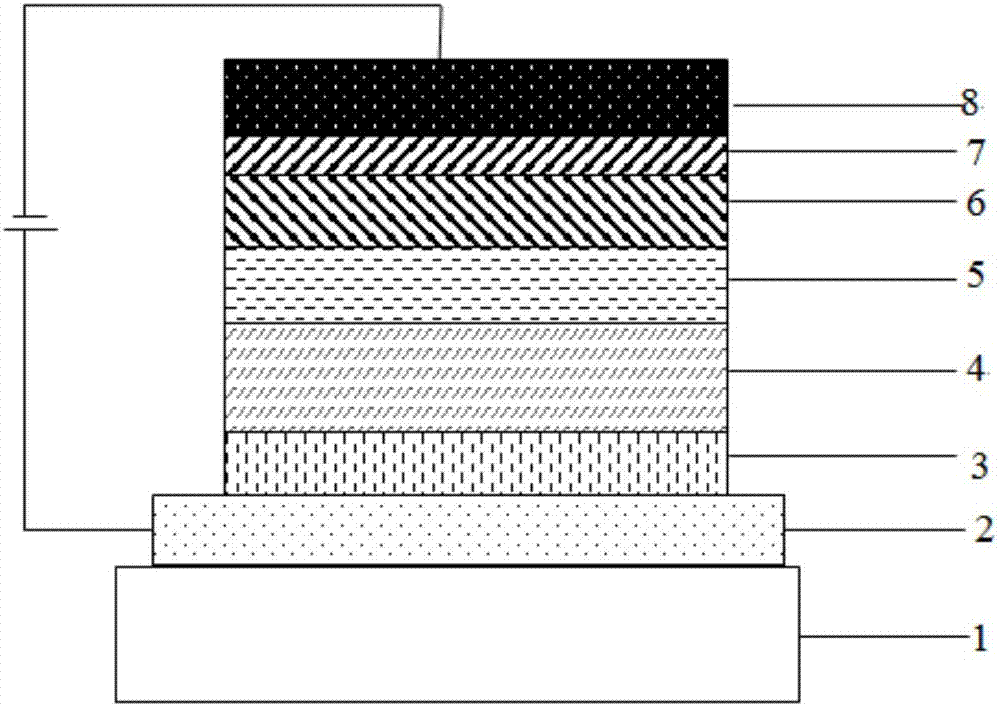
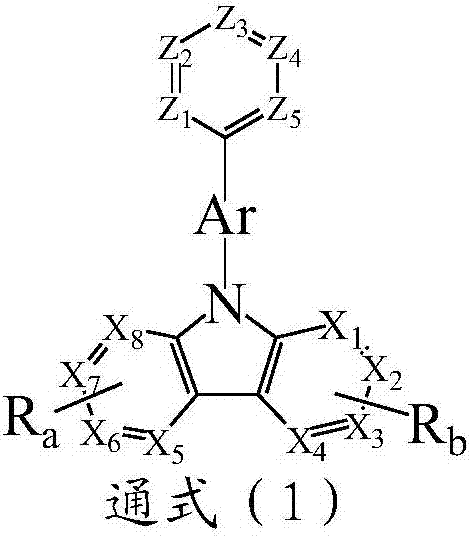
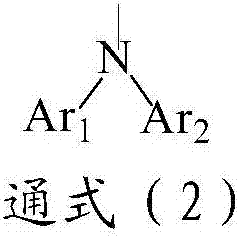
Compound taking pyridine as core and organic electroluminescence device
A technology of electroluminescent devices and compounds, which is applied in the field of semiconductors, can solve the problems of efficiency roll-off, low S1 state radiation transition rate, difficult exciton utilization rate and high fluorescence radiation efficiency, etc. Membrane properties and fluorescence quantum efficiency, effects of avoiding aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] The synthesis of embodiment 1 compound 1
[0097]
[0098] The concrete synthetic route of this compound is provided now:
[0099]
[0100] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4-(4-bromophenyl)-2,6-bis-(4-tert-butylphenyl)-pyrimidine, 0.015molN,N, N',N'-Tetra-p-benzyl-9H-carbazole-3,6-diamine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, showing no bromide remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09MPa, 85°C), After passing through a neutral silica gel column, the target product was obtained with a purity of 99.80% and a yield of 62.20%.
[0101]HPLC-MS: The theoretical value is 975.52, and the measured value is 975.68.
Embodiment 2
[0102] The synthesis of embodiment 2 compound 5
[0103]
[0104] The concrete synthetic route of this compound is provided now:
[0105]
[0106] In a 250ml four-neck flask, add 0.01mol 4-(3-bromophenyl)-2,6-bis(4-tert-butylphenyl)-pyridine, 0.015molN,N,N ',N'-Tetra-p-benzyl-9H-carbazole-3,6-diamine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, showing no bromide remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09MPa, 85°C), After passing through a neutral silica gel column, the target product was obtained with a purity of 95.76% and a yield of 58.00%.
[0107] HPLC-MS: The theoretical value is 974.53, and the measured value is 974.65.
Embodiment 3
[0108] The synthesis of embodiment 3 compound 8
[0109]
[0110] The concrete synthetic route of this compound is provided now:
[0111]
[0112] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4-(4-bromophenyl)-2-dibenzofuran-4-yl-6-phenyl-pyridine, 0.015molN,N,N ',N'-Tetra-p-benzyl-9H-carbazole-3,6-diamine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, showing no bromide remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09MPa, 85°C), After passing through a neutral silica gel column, the target product was obtained with a purity of 98.63% and a yield of 82.80%.
[0113] HPLC-MS: The theoretical value is 952.41, and the measured value is 952.52.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com