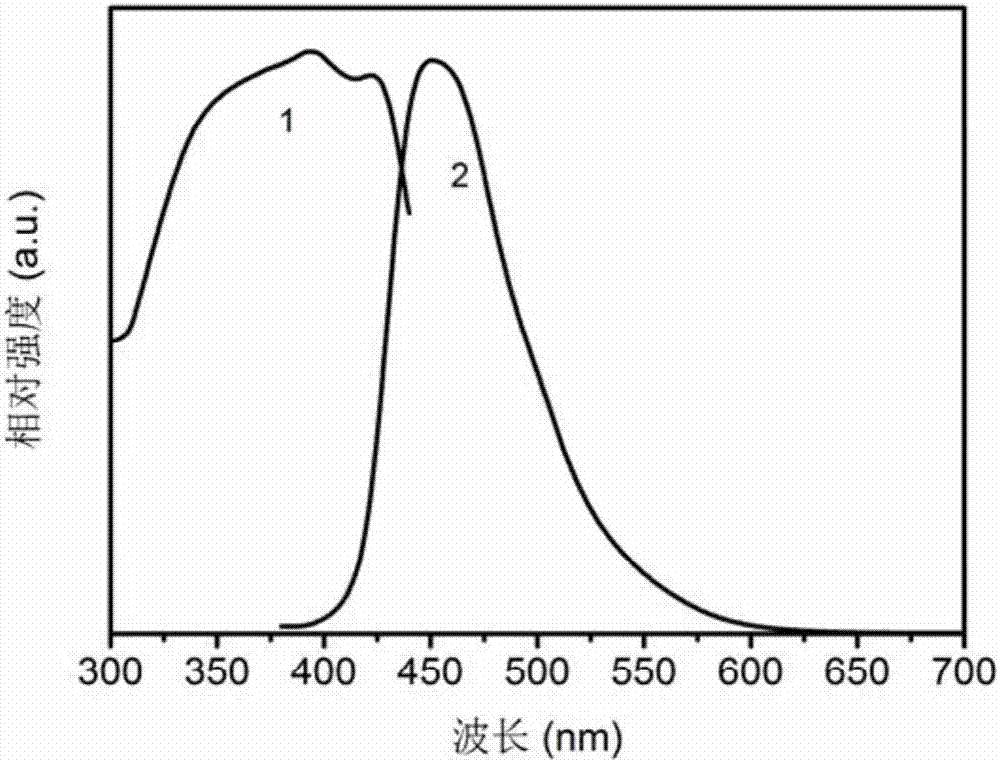
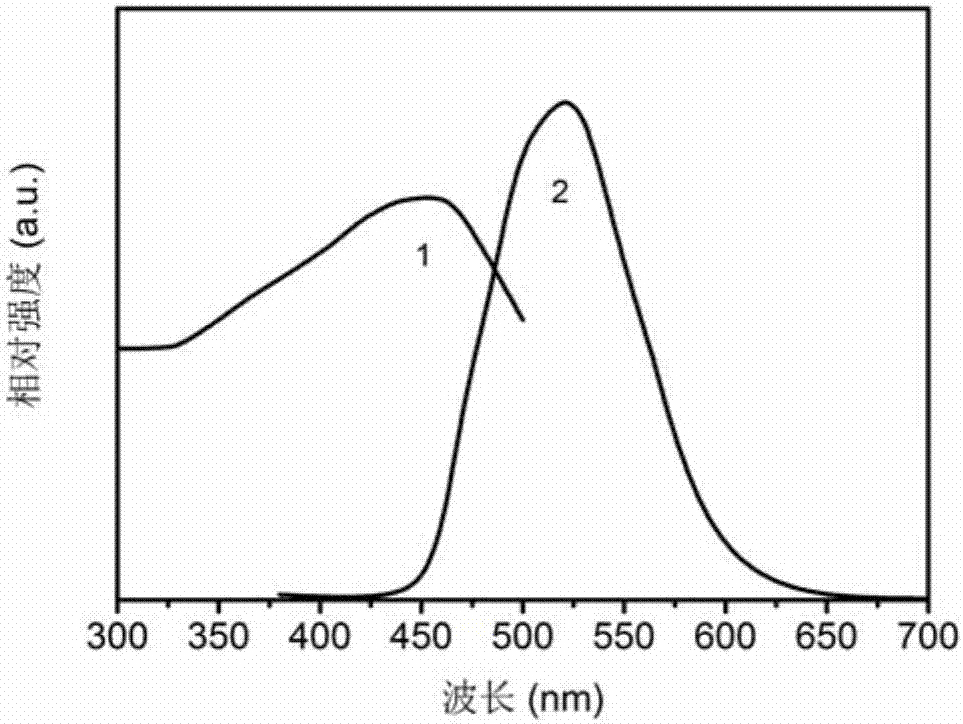
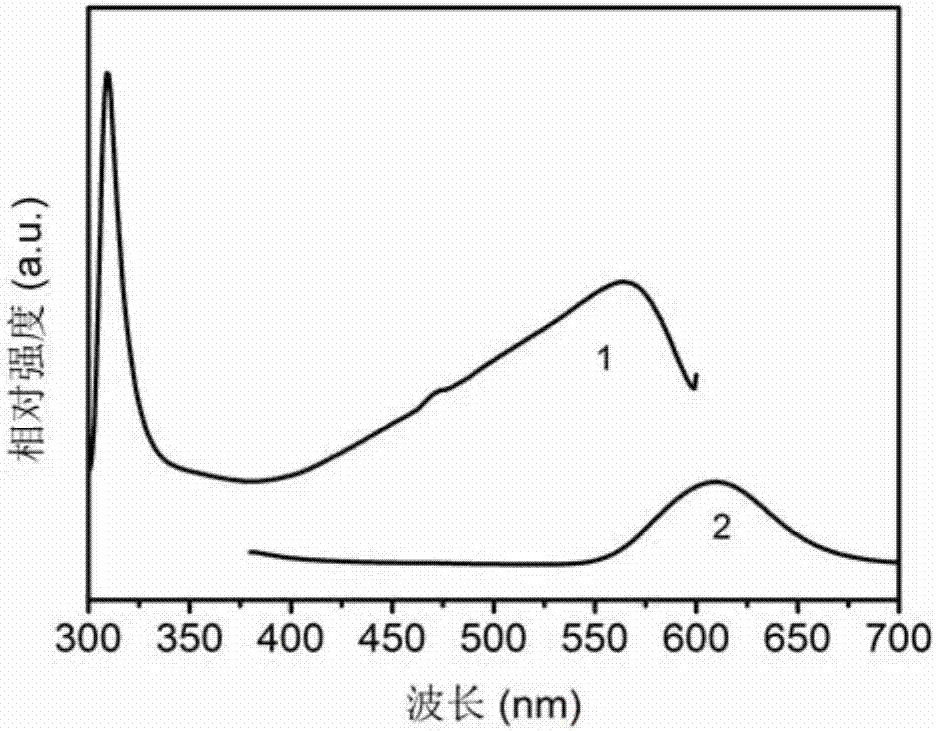
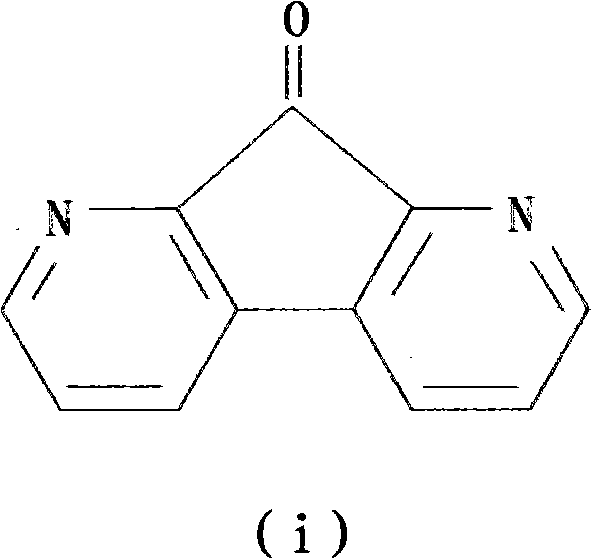
Patents
Literature
135 results about "9-fluorenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
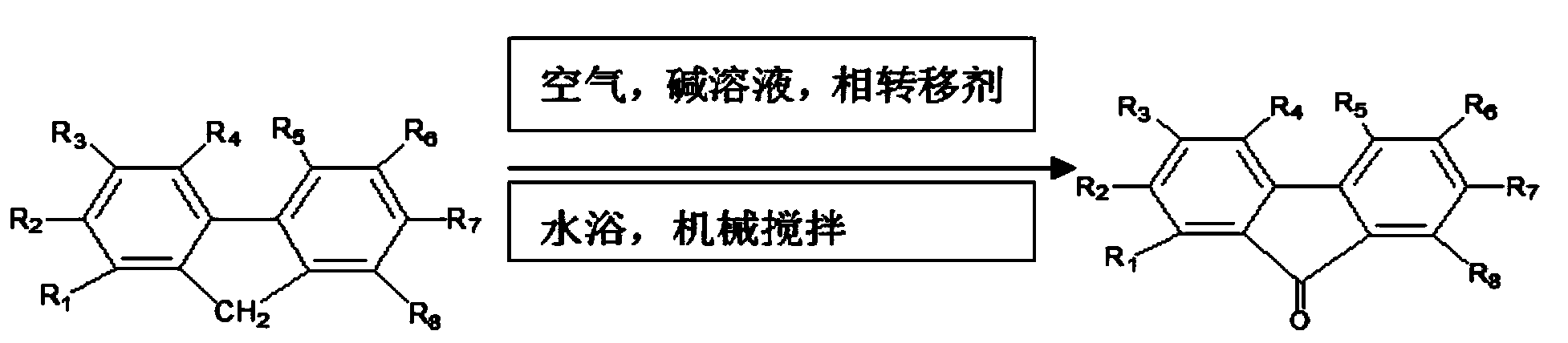
The major ethyl acetate-soluble metabolites were 9-fluorenone (62%), 9-fluorenol, and 2-hydroxy-9-fluorenone (together, 7.0%). Similarly to bacteria, Cunninghamella elegans oxidized fluorene at the C-9 position of the five-member ring to form as alcohol and the corresponding ketone.
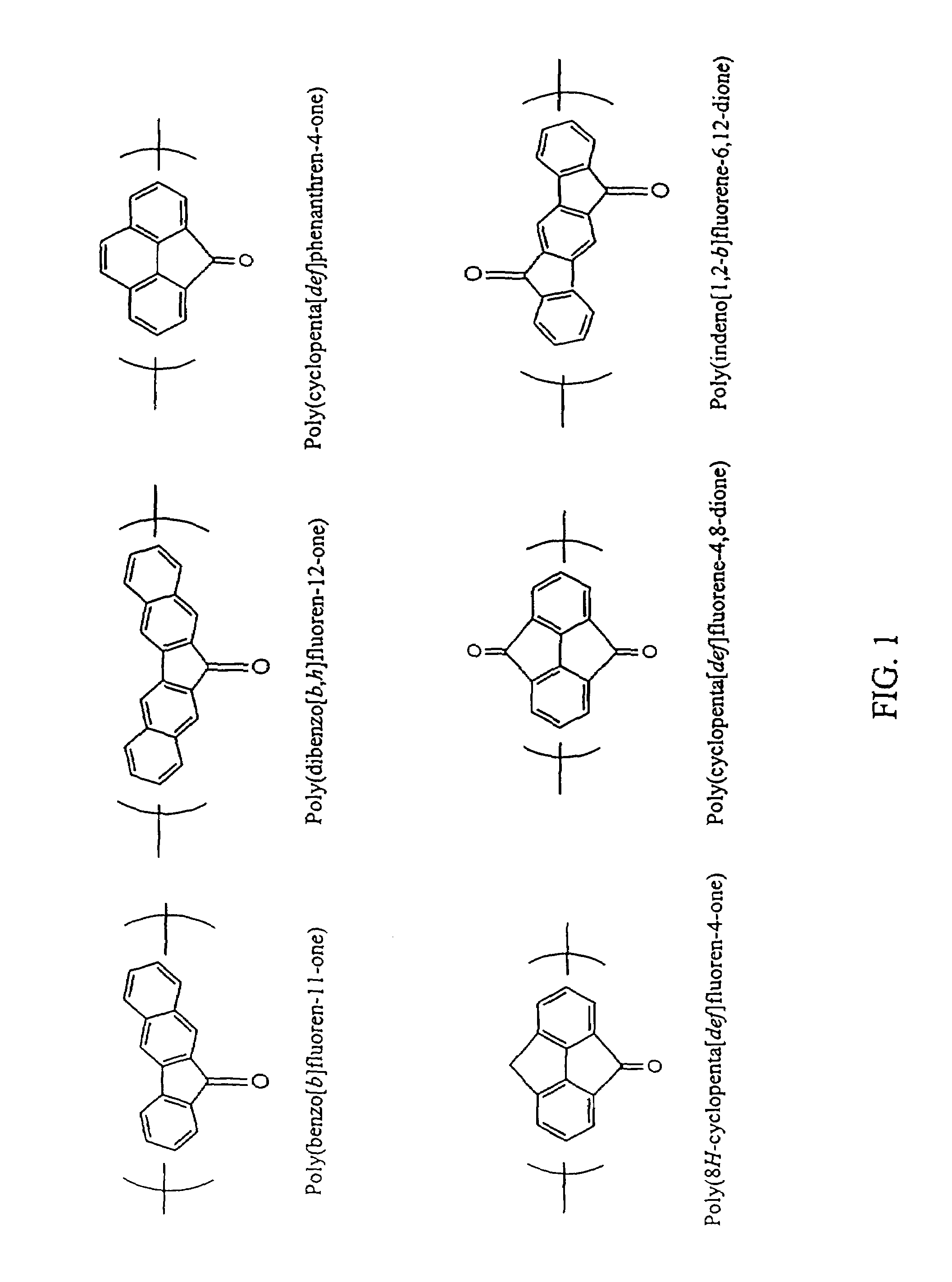
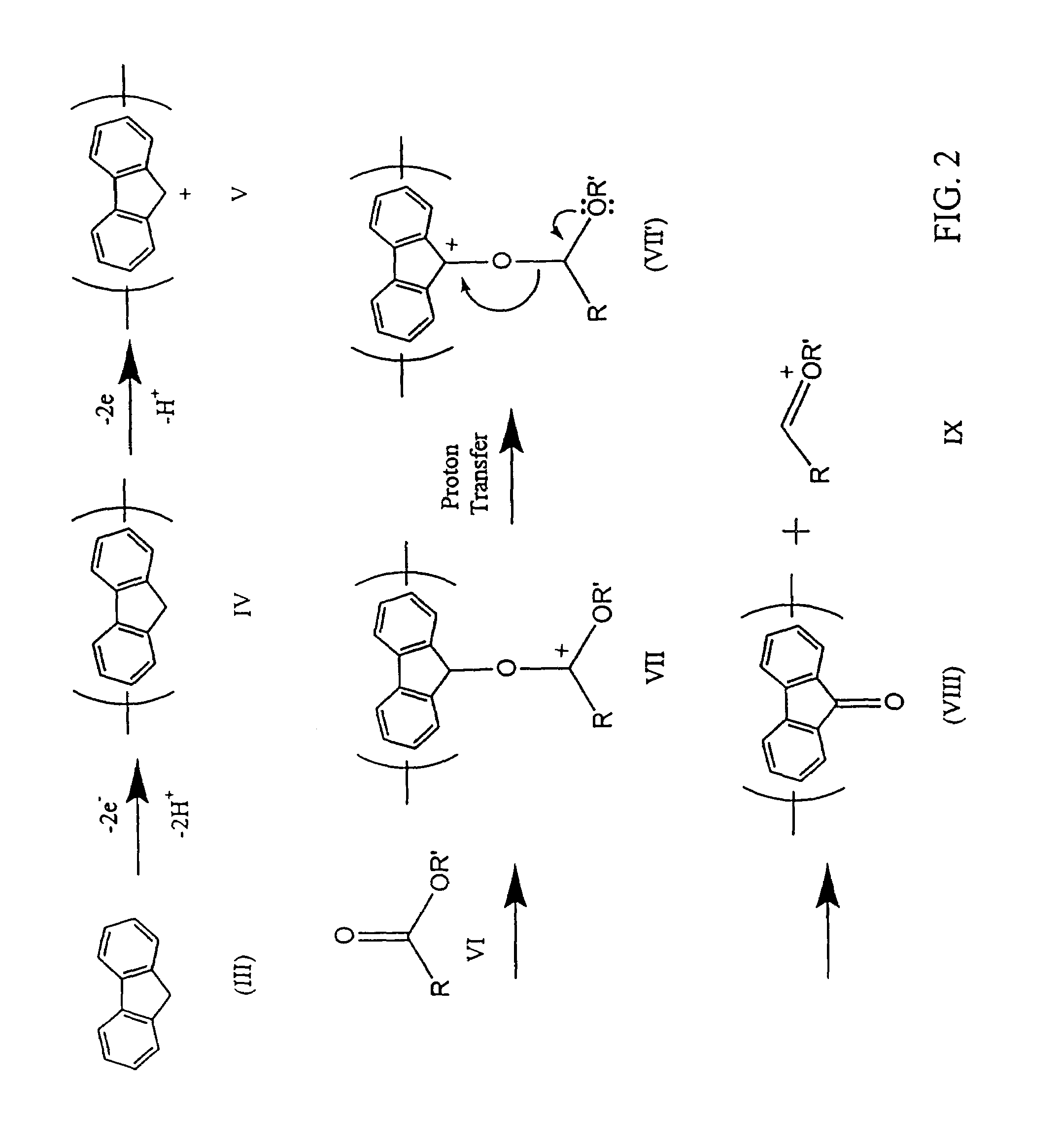
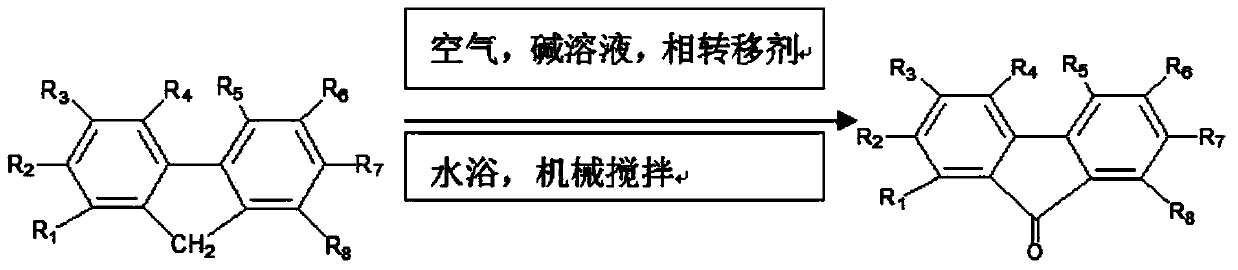
Method for preparing polymers containing cyclopentanone structures
InactiveUS7087681B2Increase productionElectrolysis componentsElectrolytic organic productionAromatic hydrocarbonOxidizing agent
A method to electrolytically polymerize aromatic hydrocarbons and oxidize cyclopentane structures within the hydrocarbons into cyclopentanone structures is disclosed including a method to electrolyze fluorene in the presence of an ester to produce poly(9-fluorenone). A method to electrolytically oxidize polymers having cyclopentane structures to polymers having cyclopentanone structures is also disclosed including a method to electrolyze poly(fluorene) to produce poly(9-fluorenone). In addition, a method to chemically oxidize polymers containing cyclopentane structures into polymers containing cyclopentanone structures is disclosed, including a method to oxidize poly(fluorene), with a chemically prepared oxidizing agent, to produce poly(9-fluroenone).
Owner:IM&T RES INC
Method for producing 9-fluorenone
ActiveCN102020543ALow costEasy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDistillationFluorenone
The invention relates to a method for producing 9-fluorenone, comprising the following steps of: (1) feeding industrial fluorine as a raw material and methylbenzene as a solvent into a reaction kettle according to the weight ratio of 1:(6-8) with quaternary ammonium salt as a catalyst; heating the mixture to 40-80DEG C; adding the catalyst after the raw material is completely dissolved at the addition of 5-20 percent of industrial fluorine; introducing the air according to 2-3m<3> / h of each kilo of industrial fluorine and reacting under the condition of 40-80DEG C for 2-5h; (2) after the reaction is ended, pouring a reactant into a distillation kettle for distillation at normal temperature, recovering the solvent and obtaining an industrial fluorine crude product from the bottom of the kettle; and (3) recrystallizing the crude product with the solvent to obtain fluorenone with the content of 99 percent. The method has the advantages of simple process flow, fewer reactants and low cost, is easy to control and can be widely used for producing the fluorenone; and the finished products prepared by the method have favorable quality.
Owner:鞍钢化学科技有限公司
Method for oxidizing fluorene to 9-fluorenone
ActiveCN1754867AIncrease profitHigh yieldOrganic compound preparationCarbonyl compound preparationWastewaterOxygen
The invention discloses a method to prepare 9-fluorenone with fluorene. Wherein, using dimethyl sulfoxide as solvent, sodium hydroxide as catalyst, oxygen as oxidant and tower filling reactor; cooling and filtering the reacted liquid to obtain 93% coarse fluorenone; distilling liquid and recycling 94% solvent and some coarse fluorenone; refining with oriented crystallization method and obtaining yellow piece 99.8% fluorenone. This method is simple, needs short reaction time, produces no waste water, and convenient to produce in industry.
Owner:SHANGHAI HUAYI ENERGY CHEM
Preparation method of 9-fluorenone
InactiveCN102391087AEasy to handleReduce pollutionOrganic compound preparationCarbonyl compound preparationDistillationGas phase
The invention relates to the technical field of production of an aromatic compound 9-fluorenone, in particular to a preparation method of the 9-fluorenone. The preparation method includes the steps as follows: 1) taking industrial fluorene, benzene series solvent, sodium hydroxide and quaternary ammonium salt, and adding the four components in a four-mouth bottle; 2) stirring the mixture in normal pressure, increasing the temperature to above 90 DEG C, reacting, and introducing air for oxidization; 3) conducting reduced-pressure distillation and allowing concentrate to crystallize, thus obtaining yellow fluorenone crystals; and 4) washing the fluorenone crystals for one time and then drying the fluorenone crystals, thus obtaining the 9-fluorenone product. The benzene series solvent is toluene or dimethylbenzene. Compared with the prior art, the preparation method has the benefits as follows: 1) the reaction temperature is low, the operation is simple and convenient and the reaction conditions are moderate; 2) the cheap sodium hydroxide is taken as a catalyst, so the cost is low; 3) the benzene series solvent can be recycled by reduced-pressure distillation, washing water is little in quantity, treatment is easy and pollution to environment is small; and 4) the gas chromatographic purity of the product is higher than 99.2%, the yield is higher than 86.7% and the production requirements are met.
Owner:SINOSTEEL ANSHAN RES INST OF THERMO ENERGY CO LTD
Method for synthesizing bisphenol fluorine by catalysis of highly acidic cation exchange resin
InactiveCN101003466ASolve pollutionHigh purityOrganic chemistryOrganic compound preparationCarboxylic acidPhenol
This invention discloses a method for synthesizing biphenol fluorine by using strongly acidic cation exchange resin as catalyst. The method comprises: adding phenol, 9-fluorenone, strongly acidic cation exchange resin, and mercapto carboxylic acid into a container, reacting at 80-120 deg.C under stirring for 4-15 h, filtering after reaction, recovering the catalyst, adding methanol aqueous solution into the filtrate, precipitating the crystals, vacuum-filtering to obtain crude biphenol fluorene, recrystallizing with organic solvent, filtering, and vacuum-drying to obtain white biphenol fluorene crystals with a purity up to 99.8%. The phenol / 9-fluorenone mol ratio is (6-15):1. The cation exchange resin is 5-30 wt. % of the reactants. The post-treatment is simple, thus solving the problems of metal corrosion and environmental pollution. Besides, the catalyst can be recycled.
Owner:HARBIN ENG UNIV
Method for preparing 9-fluorenone via four-phase transfer catalysis
ActiveCN103435463AImprove utilization efficiencyAdjustment and PurityOrganic compound preparationCarbonyl compound preparationQuaternary ammonium cationDissolution
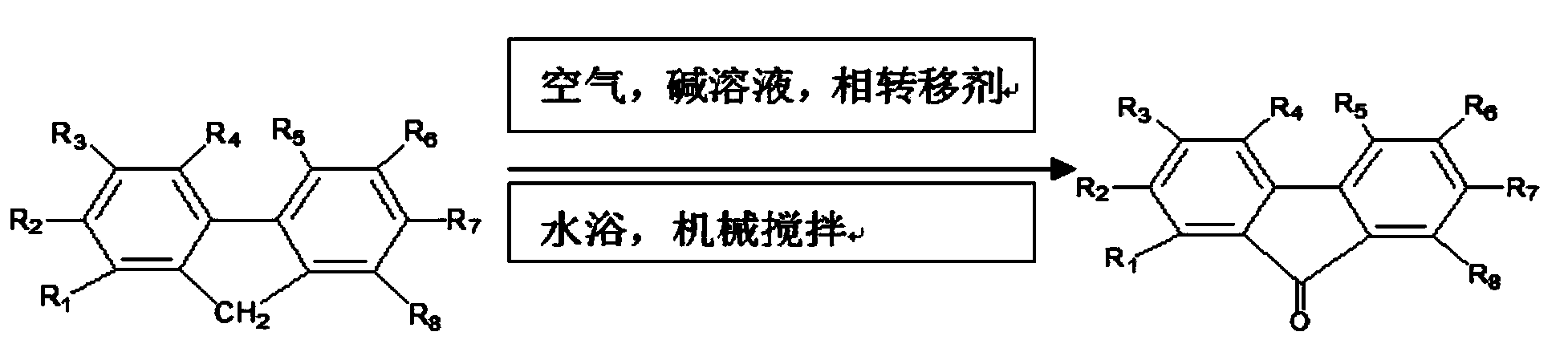
The invention discloses a method for preparing 9-fluorenone via four-phase transfer catalysis. At a lower temperature, alkali is taken as a catalyst, quaternary ammonium salt serves as a phase transfer agent, fluorene can be excessive and reacts with oxygen-containing gas in xylene in states of dissolution and suspension, and high-purity 9-fluorenone is directly obtained, a right reaction condition is selected, and the transformation rate of the fluorene can reach 100 percent. According to the invention, the recovered alkali and the dissolvent do not need to be treated complexly and particularly, reuse can be realized, and industrialization is facilitated; in addition, mixed xylene and water are taken as the dissolvent, the oxidation of the fluorene as the reaction condition of the 9-fluorenone, the selection of the catalyst alkali and phase transfer agent, and the reuse of the dissolvent and the alkali are researched, as a result, the conditions of high transmission rate of fluorene and high selective transmission of the 9-fluorenone are obtained, and a more complete compounding process is disclosed.
Owner:BAOSHUN TECH CO LTD +1
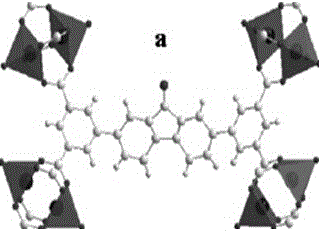
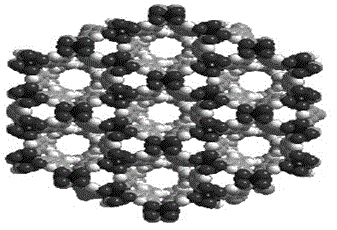
High selectivity metal organic skeleton material and preparation method thereof
InactiveCN105713017AImprove adsorption capacityImprove physical and chemical propertiesOther chemical processesChemical industryCooking & bakingCarbon nanotube
The invention discloses a high selectivity metal organic skeleton material and a preparation method thereof. The preparation method comprises the following steps: adding an organic ligand namely 2,7-bis(3,5-dibenzoic acid)-9-fluorenone, carbon nanotubes, and a copper source into an amine solvent and deionized water, evenly stirring in an enclosed container; then adding a nitric acid solution, evenly mixing, transferring the enclosed container to a baking oven to carry out crystallization; subjecting the obtained blue-green hexagonal crystals to multi-stage solvent extraction and solubilization treatments with N,N'-dimethyl formamide and methanol in sequence, and performing vacuum drying to obtain the pure metal organic skeleton material. The metal organic skeleton material comprises a secondary structural unit, which is formed through coordination between a Cu2(COO)4 structural unit and oxygen atoms of carboxylic acid. The secondary structural units are linked to each other so as to form a three dimensional channel structure. The provided high selectivity metal organic skeleton material is especially suitable for selective adsorption separation of mixed gas (acetylene, ethylene, and ethane), the operation conditions are mild, and the service life is long.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method for 9-fluorenone
ActiveCN103435456AReduce energy consumptionImprove conversion rateOrganic compound preparationCarbonyl compound preparationNitrogenous heterocyclic compoundQuinoline
The invention discloses a preparation method for 9-fluorenone. According to the method, industrial fluorene (the purity is more than 95 percent) is taken as a raw material, alkali is taken as a catalyst, a heterocyclic compound containing nitrogen and water are used as solvent and quaternary ammonium salt is taken as a phase transfer agent; under the lower temperature and stirring condition, fluorene and gas containing oxide react and the fluorene is converted into 9-fluorenone; by selecting proper reaction conditions, the conversion rate of the fluorene can reach 100 percent. The recovered alkali and solvent in the invention can be recycled without a special complex treatment process and benefit the industrialization. According to the preparation method disclosed by the invention, quinoline and isoquinoline as well as a mixer of the quinoline and the isoquinoline are taken as the solvent and the reaction condition that the fluorene is oxidized into the 9-fluorenone, the selection of the alkali as the catalyst and the phase transfer agent and the recycling of the solvent and the alkali are researched to obtain the conditions that the fluorene is converted into the 9-fluorenone at high conversion rate and high selectivity; and a complete synthetic process is provided.
Owner:BAOSHUN TECH CO LTD +1
Bisphenol monomer containing bi-tert-butyl and fluorenyl structure, and preparation method and application thereof
ActiveCN103274908AThe synthetic route is simpleHigh yieldOrganic chemistryOrganic compound preparationPolymer scienceTert butyl phenol
The invention relates to a bisphenol monomer containing a bi-tert-butyl and fluorenyl structure. The bisphenol monomer is 9, 9-bi(4-hydroxyl-3-tert-butyl phenyl) fluorine. The monomer is simple in a synthetic route, high in yield, easy to purify, stable at the room temperature and can be used for preparing fluorine-contained polyarylether. The bisphenol monomer containing the bi-tert-butyl and fluorenyl structure is prepared by the following steps of: reacting reactants 2-tert butyl phenol and 9-fluorenone in the presence of an acid catalyst, transferring a product into a mixed solvent of ethanol and water, carrying out suction filtering, and further recrystallizing to obtain the target product. The bisphenol monomer containing the bi-tert-butyl and fluorenyl structure is used for preparing the fluorine-contained polyarylether. The prepared polyarylether has good dissolving film forming property, excellent thermal property and low dielectric constant.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
Bisphenol fluorene synthesizing process catalyzed with solid magnetic super acid
InactiveCN1986510AEasy to recycleReduce pollutionPhysical/chemical process catalystsOrganic chemistryCarboxylic acidPhenol
The bisphenol fluorine synthesizing process includes mixing phenol, 9-fluorenone, solid magnetic super acid and mercapto carboxylic acid, with phenol / 9-fluorenone molar ratio of 6-12 to 1, mercapto carboxylic acid / 9-fluorenone weight ratio of 0.02-0.50 to 1 and solid magnetic super acid accounting for 5-15.0 % of total reactant weight, to react at 80-110 deg.c via stirring for 6-16 hr; magnetically separating after reaction to adsorb solid magnetic super acid to the bottom of reactor, transferring the hot reaction product to some container, washing the reactor with hot water solution of methanol, transferring the washed liquid to the container, cooling the container to separate out crystal, filtering, drying, recrystallizing the filter cake in organic solvent and stoving to obtain bisphenol fluorine crystal. The present invention has mild reaction condition and product purity as high as 99.2 %, and may be used widely in the phenol and 9-fluorenone condensation reaction.
Owner:HARBIN ENG UNIV
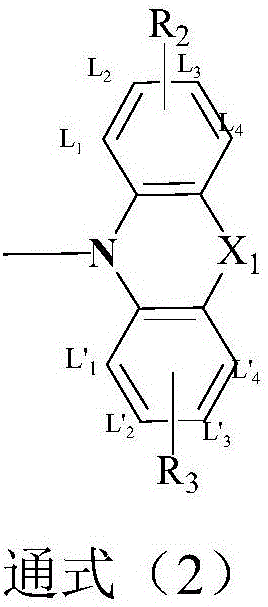
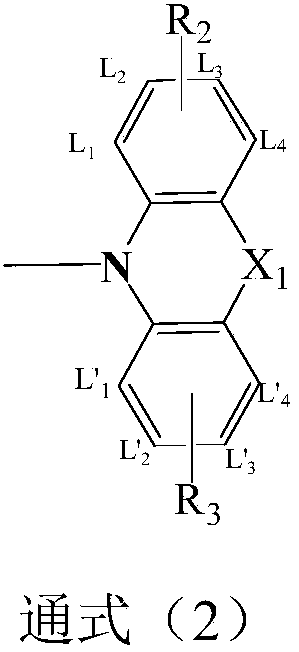
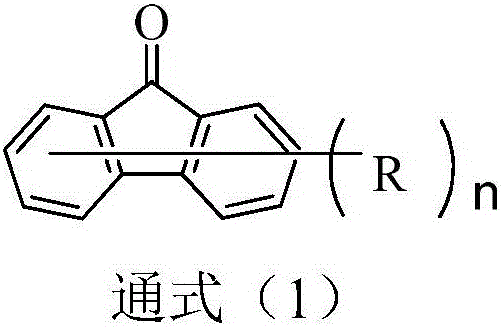
Compound based on monosubstituent-9-fluorenone and application thereof
ActiveCN106220645ADestroy crystallinityInhibit aggregationOrganic chemistrySolid-state devicesFluorenoneCrystallinity
The invention discloses a compound based on monosubstituent-9-fluorenone and application thereof. The compound uses monosubstituent-9-fluorenone as a mother nucleus and allows a single side to be connected with a heteroaromatic group, so crystallinity of molecules is destroyed, aggregation of molecules is prevented, and good film forming ability is obtained. The compound is used as a luminescent layer material for an organic light-emitting diode and has good photoelectric performance.
Owner:JIANGSU SUNERA TECH CO LTD
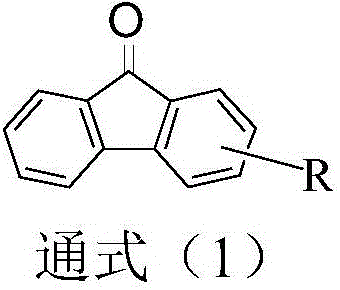
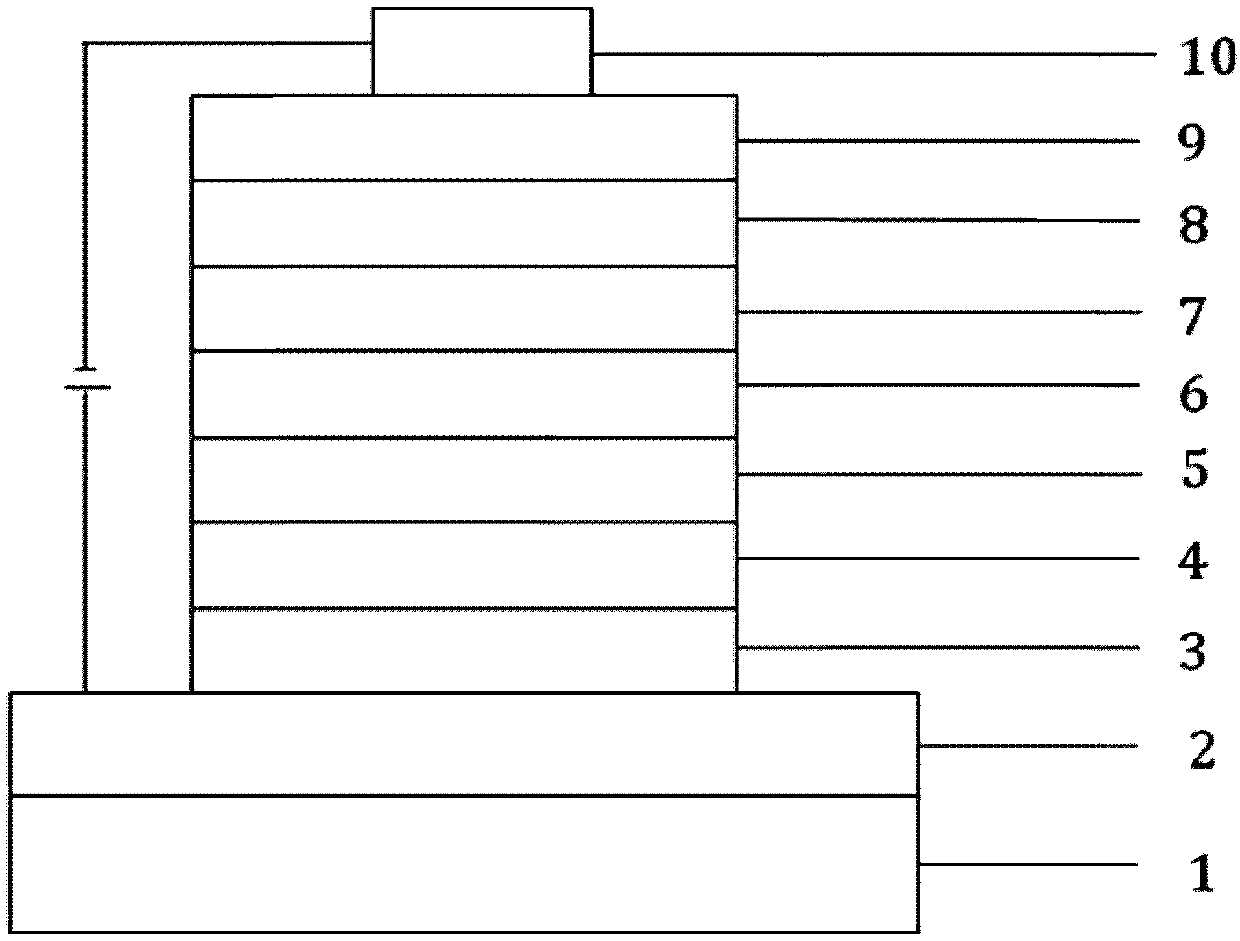
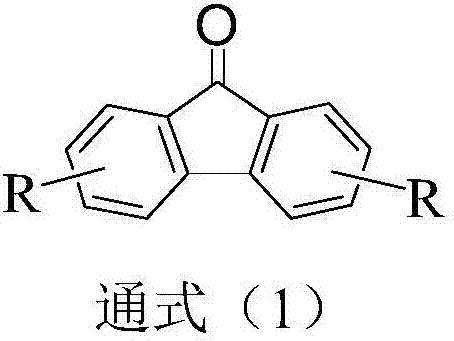
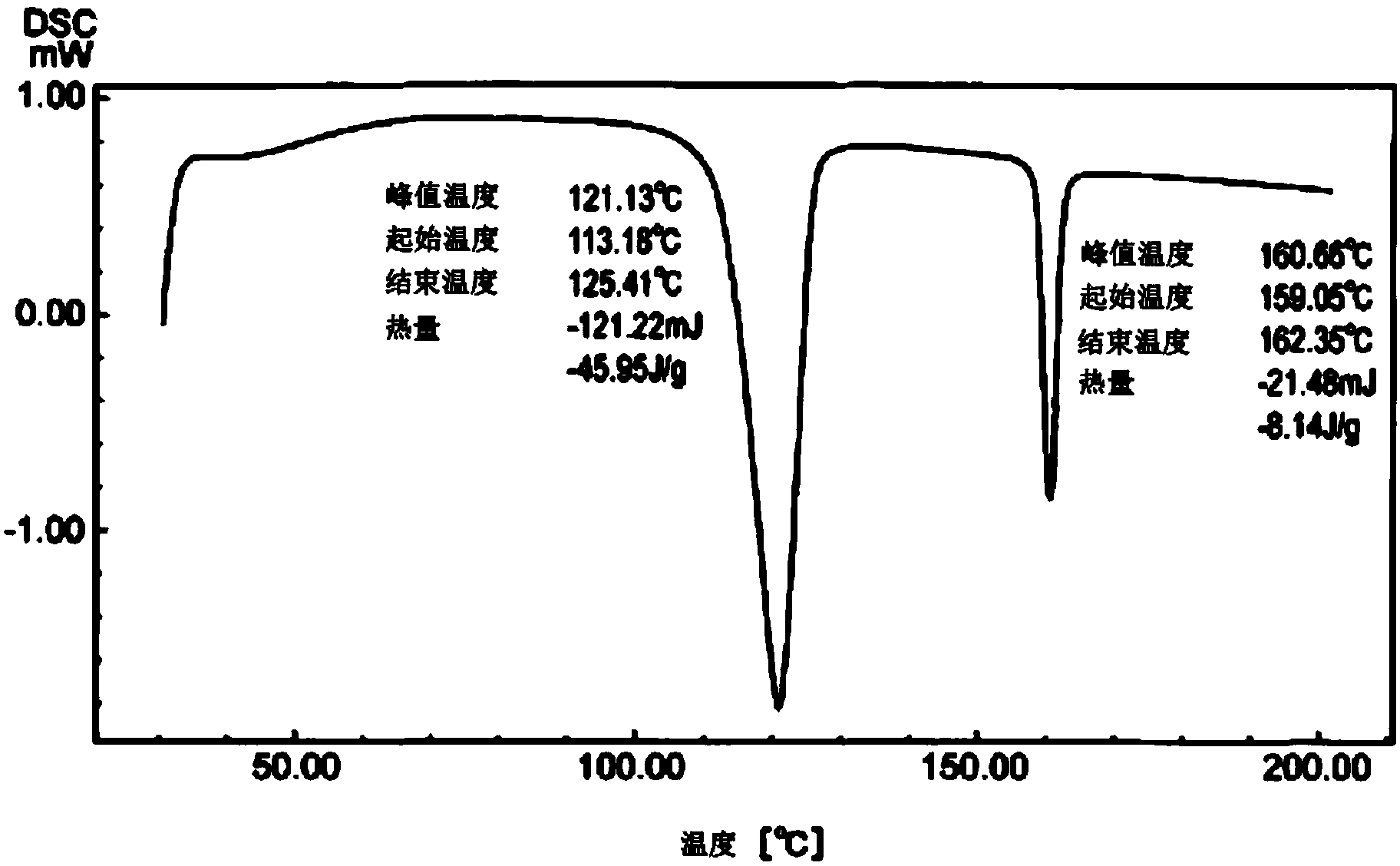
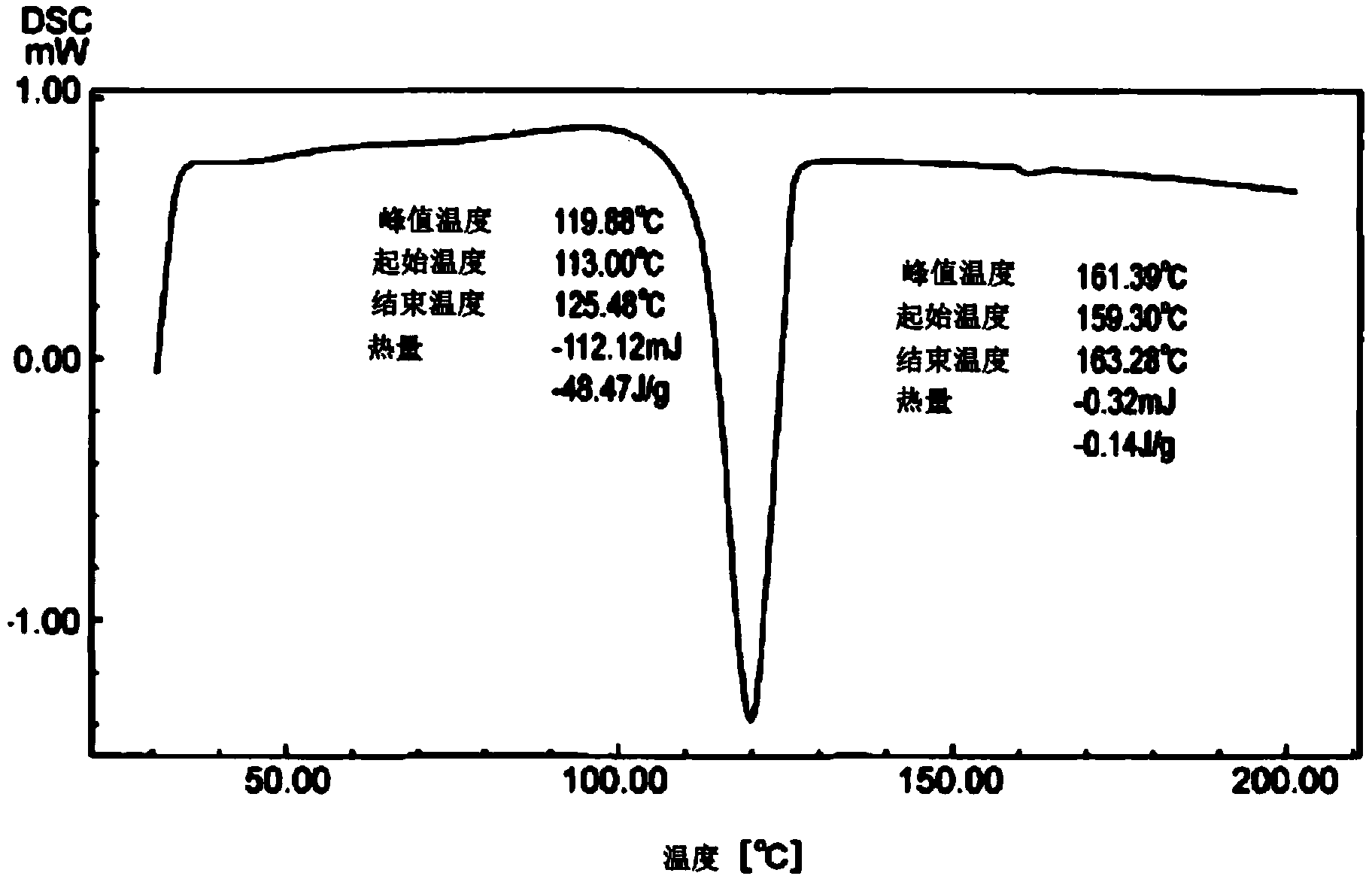
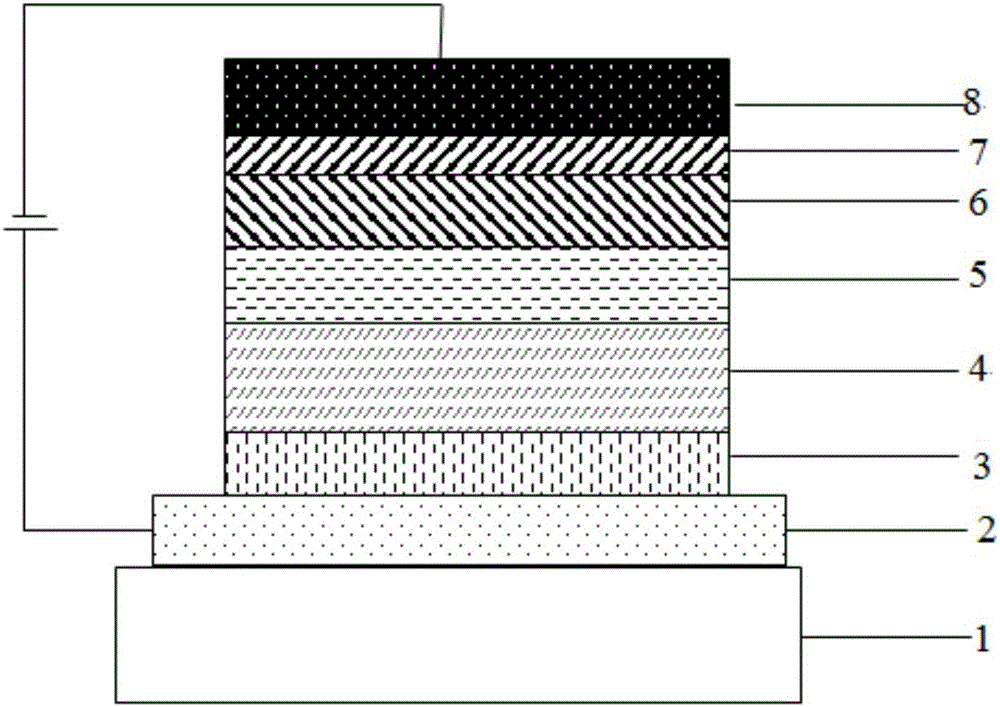
Dual-substituent-group-9-fluorenone compound-containing organic light-emitting device and application thereof
ActiveCN107046100AEasy to achieve anti-intersystem crossingAnti-intersystem crossingSolid-state devicesSemiconductor/solid-state device manufacturingEnergy transferChemical compound
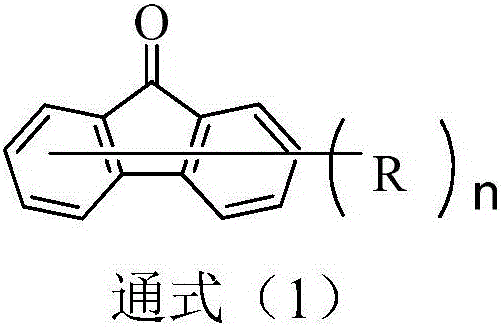
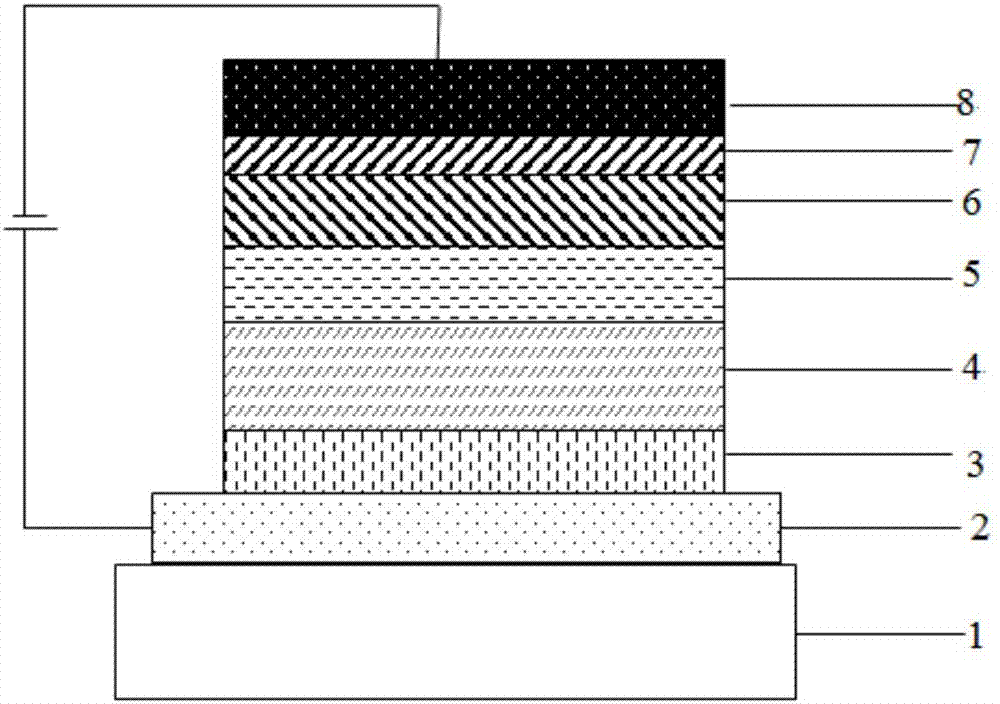
The invention discloses a dual-substituent-group-9-fluorenone compound-containing organic light-emitting device and an application thereof. The device comprises a hole transport layer, a light emitting layer and an electron transfer layer; the light emitting layer of the device adopts the material of the dual-substituent-group-9-fluorenone-group-containing compound which is as shown in the general formula (1). The adopted dual-substituent-group-9-fluorenone-group compound has relatively low triplet state and singlet state energy difference, so that energy transfer between host-guest materials can be realized easily; therefore, energy which is originally dissipated in a heat form can be obtained and utilized easily, thereby obtaining high efficiency of a device more easily; and furthermore, when a fluorescent material is selected as a doping material, the light emitting radiation of the doping material can be obtained more easily, thereby achieving long service life of the material more easily.
Owner:JIANGSU SUNERA TECH CO LTD
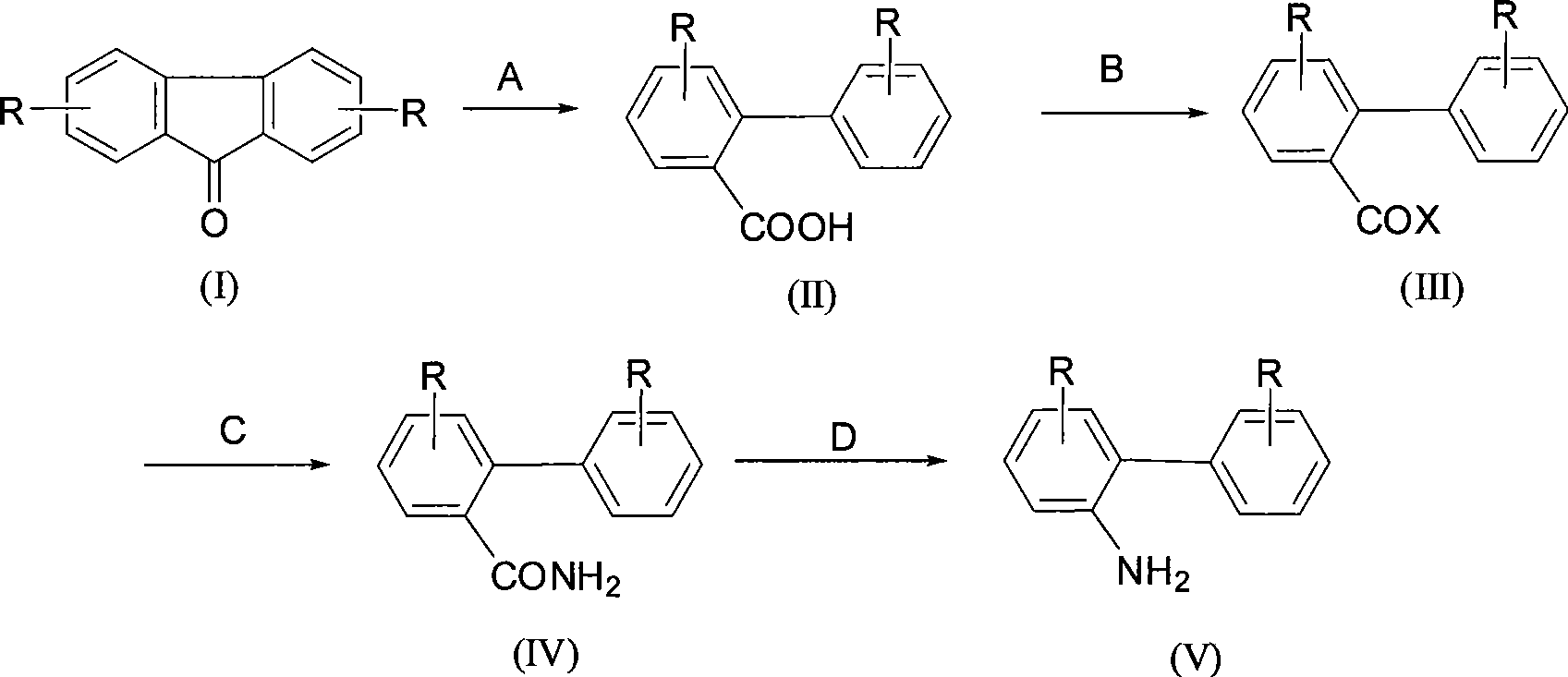
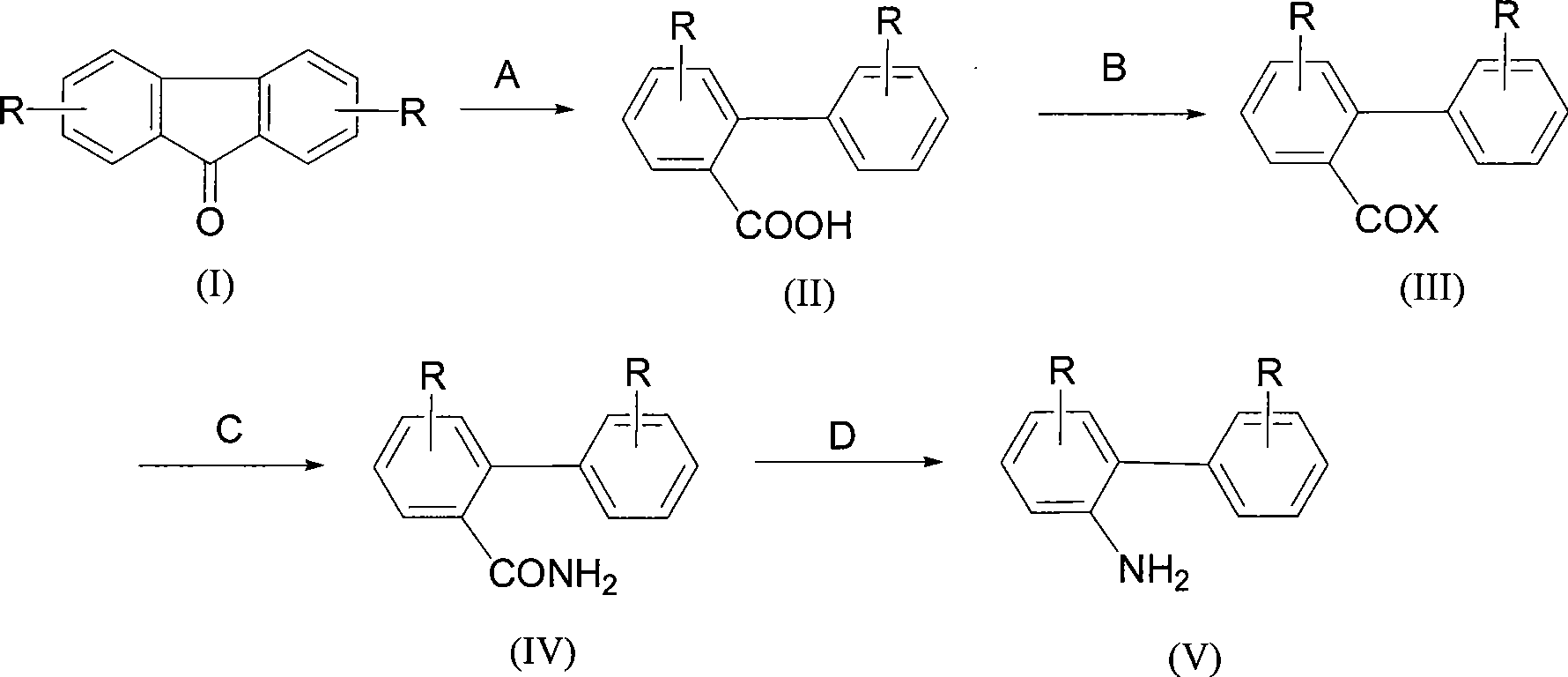
Synthesis of 2-aminobiphenyl compounds
InactiveCN101367736AAtom utilization is highMild reaction conditionsOrganic compound preparationAmino compound preparationSynthesis methodsFluorenone
The present invention relates to a synthesis method of 2-phenylaniline compounds as shown in the formula (V). The synthesis method comprises the following steps: substituted 9-fluorenone as shown in the formula (I) reacts under alkaline conditions to prepare a compound (II); the compound (II) and acylation agent perform acyl halogenation reaction to prepare a compound (III); the compound (III) performs ammoniation reaction to prepare a compound (IV); and the compound (IV) performs Hofmann degradation reaction to prepare the compound (V). The present invention discloses a novel synthesis method of the 2-phenylaniline compounds, and the present invention has the advantages of mild reaction conditions, easy operation, high utilization rate of atoms, high reaction yield, low production cost, suitability for industrial production, high implementation value and the socioeconomic benefits.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing 9-fluorenone through using industrial fluorene
ActiveCN103467264AReduce energy consumptionOrganic compound preparationChemical recyclingSolventFluorenone
A method for preparing 9-fluorenone through using industrial fluorene is characterized in that a raw material industrial fluorene (having a purity of above 95%) reacts with an oxygen-containing gas at a low temperature under stirring in a solvent comprising toluene and water under the action of an alkali as a catalyst and a quaternary ammonium salt as a phase transfer agent to form 9-fluorenone. The fluorene conversion rate can reach 100% under appropriate reaction conditions. The alkali and solvent recovered in the invention can be recycled without special complex treatment processes, so it is beneficial for industrialization; and in the invention, the recycle of the catalyst alkali, the phase transfer agent quaternary ammonium salt and the solvent, a recrystallization technology and the like are researched, pure fluorenone is obtained, and the complete preparation method of the 9-fluorenone is provided.
Owner:BAOSHUN TECH CO LTD +1
Oxidation preparation method for 9- fluorenone compound from fluorine compound
InactiveCN1962597AReduce pollutionMild reaction conditionsOrganic compound preparationCarbonyl compound preparationPotassium hydroxideFluorenone
The invention discloses a preparing method of 9-fluorenone compound through oxidizing fluorene compound, which comprises the following steps: dissolving fluorene compound in the tetrahydrofuran; adding potassium hydroxide; setting the weight rate of fluorene compound and tetrahydrofuran at 1:4-6 with the molar rate of fluorene compound and potassium hydroxide at 1:0.5-2.5; stirring under normal temperature and pressure; oxidizing in the air; reacting for 1-8h; filtering; distilling; washing; drying; obtaining the product with producing rate at 98-99% and purity at 99-99.5%.
Owner:SHANXI UNIV
Method for preparing 2-bromo-7-nitrofluorenone
InactiveCN101514161AReduce pollutionMild reaction conditionsNitro compound preparationAcetic acidSolvent
A method for preparing 2-bromo-7-nitrofluorenone comprises the followings steps: carrying out oxidation reaction on fluorene to obtain 9-fluorenone; carrying out bromination reaction on the 9-fluorenone to obtain 2-bromofluorenone; adding 2-bromofluorenone and water into a reactor by the weight ratio of 1:7-9; blending and heating the mixture; when the temperature is 80-90 DEG C, dripping the mixed acid of nitric acid and sulphuric acid rapidly; reacting for 2.5-4h under reflux, wherein the mol ratio of 2-bromofluorenone to nitric acid and sulphuric acid is 1:25-35:30-40; adding water for quenching reaction; filtering; re-crystallizing the obtained solid with methanol and glacial acetic acid; and drying the crystal to obtain the 2-bromo-7-nitrofluorenone. In the bromination and nitrification processes, water is used as solvent. The invention has moderate reaction conditions, simple operation, high total yield (above 83%), low cost, less environmental pollution and easy achievement of industrialization.
Owner:SHANXI UNIV
Porous organic polymer framework material and preparation method and application thereof
ActiveCN106496530AEasy to prepareMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkDimethyl acetal
The invention discloses a porous organic polymer framework material and a preparation method and application thereof, and belongs to the technical field of chemical and new materials. The porous organic polymer framework material connected by using methylene is obtained under the catalysis of a lewis acid catalyst by using 2,7-di(nitrogen-carbazolyl)-9 fluorenone as a structural unit and formaldehyde dimethyl acetal as a cross-linking agent, and the porous organic polymer framework material can be applied to selective imine synthesis from photocatalysis of organic amine or selective sulfoxide synthesis from photocatalysis of thioether. The porous organic polymer framework material has the characteristics of simple operation, mild reaction conditions, wide applicability and the like.
Owner:JILIN UNIV
Organic electroluminescent material with 9-fluorenone as core and application thereof
ActiveCN106467529ADestroy crystallinityInhibit aggregationOrganic chemistrySolid-state devicesOrganic electroluminescenceLight-emitting diode
The invention discloses an organic electroluminescent material with 9-fluorenone as a core and an application thereof. With the 9-fluorenone as the core, molecules of the compound are not liable to crystallize and aggregate and the compound has excellent film forming property. The compound is used on organic light emitting diodes as a luminescent layer material. The compound has excellent photoelectric performance and can satisfy application requirements of panel manufacturers better.
Owner:JIANGSU SUNERA TECH CO LTD
Method for producing 9-fluorenone by oxidizing fluorene
InactiveCN102701936ALow toxicityHigh purityOrganic compound preparationCarbonyl compound preparationPotassium hydroxideFluorenone
The invention discloses a method for producing 9-fluorenone by oxidizing fluorine. The method comprises the following steps of: dissolving industrial fluorine as a raw material in to a halogenated hydrocarbon solvent at the temperature of 30-80 DEG C, adding sodium hydroxide and a quaternary ammonium salt which are cheaper than potassium hydroxide for serving as catalysts, reacting in a reaction kettle for 0.5-5 hours, separating a liquid, and distilling an organic phase under reduced pressure for removing the solvent to obtain an intermediate substance; adding an intermediate and an alcohol solvent into the reaction kettle, heating to 40-80 DEG C for dissolving, introducing air at the speed of 1-10m<3> / h into every kilogram of industrial fluorine, and reacting for 0.5-5 hours; and after the reaction, removing the solvent under reduced pressure, cooling and crystallizing simultaneously, and filtering a crystal product to obtain fluorenone. The equipment investment is low, process operation is easy, the solvent can be recycled, and cost is effectively saved; an obtained fluorenone product has high purity and high yield; in an entire process, an industrial grade organic solvent is adopted, so that operation and running costs are low; and an entire device is controlled automatically, so that operation conditions are improved, and the product quality stability is ensured simultaneously.
Owner:卫宏远
Compound based on disubstituted-9-fluorenone and application thereof
ActiveCN107056701ADestroy crystallinityInhibit aggregationOrganic chemistrySolid-state devicesOLED9-fluorenone
The invention discloses a compound based on disubstituted-9-fluorenone and application thereof. The compound takes disubstituted-9-fluorenone as a mother nucleus, and two sides of disubstituted-9-fluorenone are connected with two aromatic heterocyclic groups, so that the crystallization of molecules is destroyed, aggregation between the molecules is avoided, and excellent film forming property is achieved. When applied to an OLED as a luminescent layer material, the compound has favorable photoelectric property.
Owner:JIANGSU SUNERA TECH CO LTD
Method for producing 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene, crystal of said compound, and method for producing said crystal
InactiveCN104144904AQuick responseResponse speed is sufficientEther separation/purificationOrganic compound preparationFluorenoneButanol
A method for producing 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene, which can be used for obtaining 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene having a high static bulk density and a low melting point, and is characterized in that 9-fluorenone is reacted with 2-phenoxy ethanol in the presence of methanesulfonic acid and the resultant product is purified using butanol.
Owner:HONSHU CHEM INDAL
Copper based metal organic skeleton material and preparation method thereof
InactiveCN105709692AImprove adsorption capacityLarge specific surface areaOther chemical processesDispersed particle separationVacuum dryingAmine solvent
The invention discloses a copper based metal organic skeleton material and a preparation method thereof. The preparation method comprises the following steps: dissolving an organic ligand namely 2,7-bis(3,5-dibenzoic acid)-9-fluorenone and a copper source into an amine solvent and deionized water, evenly stirring in a hermetical space; then adding a nitric acid solution, evenly mixing, then transferring the enclosed container to a baking oven to carry out crystallization; subjecting the obtained blue-green hexagonal crystals to multi-stage solvent extraction and solubilization treatments with N,N'-dimethyl formamide and methanol in sequence, and performing vacuum drying to obtain the pure metal organic skeleton material. The metal organic skeleton material comprises a secondary structural unit, which is formed through coordination between a Cu2(COO)4 structural unit and oxygen atoms of carboxylic acid. The secondary structural units are linked to each other so as to form a three dimensional channel structure. The provided copper based metal organic skeleton material is especially suitable for selective adsorption separation of mixed gas (acetylene, ethylene, and ethane), the operation conditions are mild, and the service life is long.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method and application of Schiff base derivative based on tricarbazole
ActiveCN104230943AHigh selectivityHigh sensitivityOrganic chemistryFluorescence/phosphorescenceOrganic solar cellOrganic field-effect transistor
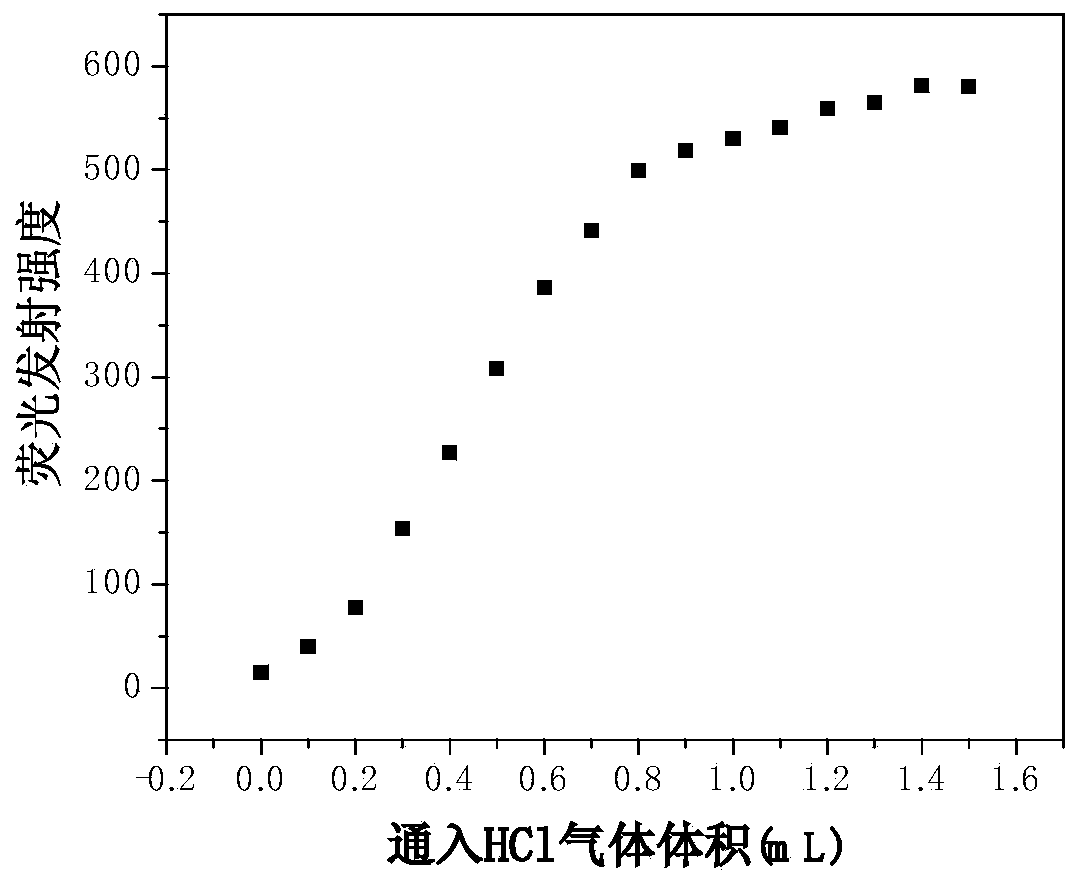
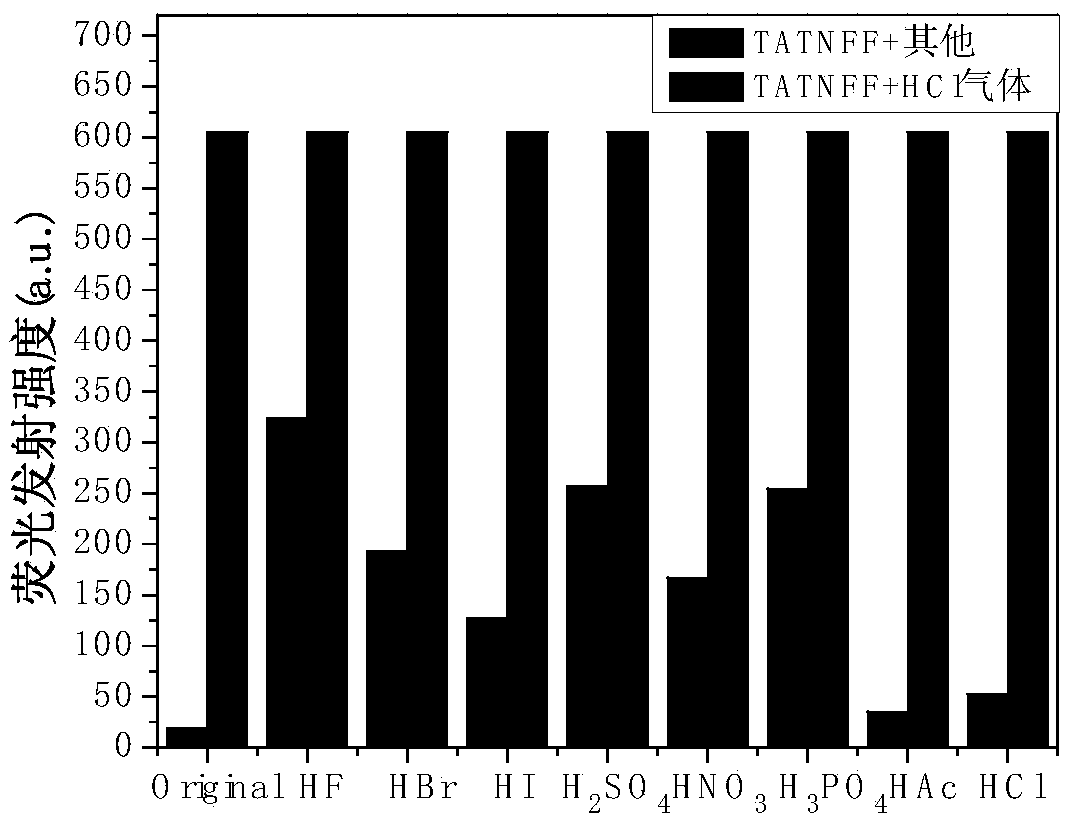
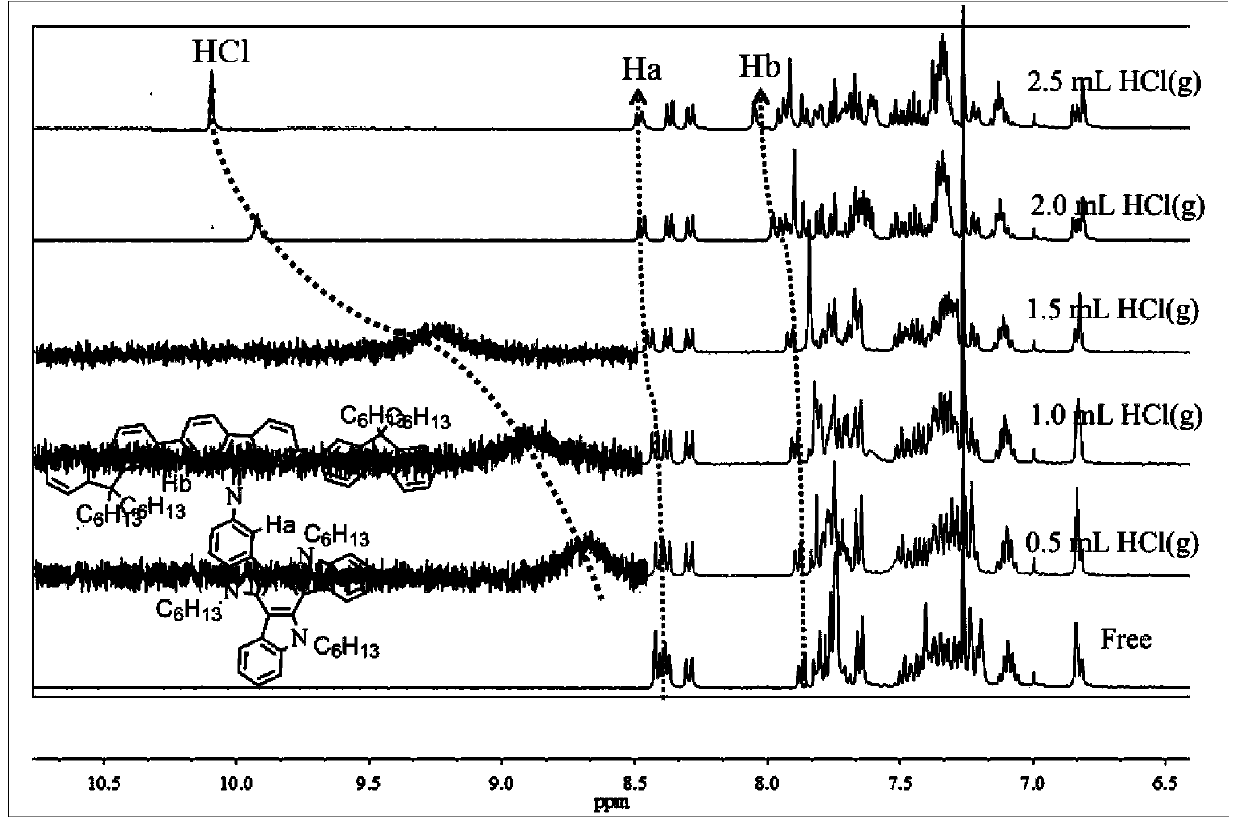
The invention discloses a Schiff base derivative material based on tricarbazole as well as a preparation method and an application method of the Schiff base derivative material. The preparation method comprises the following steps: reducing 3-nitryl tricarbazole into 3-amino-tricarbazole; then, reacting the 3-amino-tricarbazole with 2,7-dibromo-9-fluorenone to synthesize a compound II; and finally, preparing a compound I from the compound II and different fluorophores Ar by virtue of Suzuku reaction. The Schiff base derivative has good detecting performance on an HCl gas, and can be used as a small-molecule fluorescent probe. The compound is simple to prepare, the intermediate cost is low, the reaction process is easy to control; the Schiff base derivative is easy to separate, high in yield and high in purity, and has potential application value in sensors, electroluminescent devices, organic solar cells, organic field effect transistors, and the like. The compounds I and II are as shown in the specification.
Owner:NANJING UNIV OF POSTS & TELECOMM
Method for preparing 9-fluorenone through fluorene
ActiveCN103787858AReduce entrainmentThe synthesis process is simpleOrganic compound preparationCarbonyl compound preparationReaction temperatureFluorenone
Provided is a method for preparing 9-fluorenone through fluorine. The industrial fluorine is adopted as raw materials, the 9-fluorenone and water are adopted as solvents, alkali is adopted as catalysts, quaternary ammonium salt is adopted as phase transfer agents, oxygen-containing gas is piped in as oxidizing agents, the 9-fluorenone is synthetised at the reaction temperature of 70 DEG C to 83 DEG C, and the raw materials of the fluorine are added at one time or constantly replenished along with the reaction. The products, 9-fluorenone, are adopted as solvents, the process of solvent fluorenone separation, recycling and the like is eliminated, reaction liquid separation and 9-fluorenone purification are operated in a coupling mode, and the 9-fluorenone synthetic process is greatly simplified. The alkali, the quaternary ammonium salt and the by-product, water, are all recycled. Through the fluorine replenishing feeding mode, the utilization efficiency of a reactor is improved.
Owner:BAOSHUN TECH CO LTD +1
Method for preparing high-purity 9-fluorenone from high-purity fluorene
ActiveCN103804162ANo side effectsImprove utilization efficiencyOrganic compound preparationCarbonyl compound preparationOrganic solventBoiling point
The invention discloses a method for preparing high-purity 9-fluorenone from high-purity fluorene. The high-purity 9-fluorenone is prepared by using high-purity fluorene as a raw material, alkali as a catalyst, an aromatic organic solvent and water as a solvent and a quaternary ammonium salt as a phase transfer agent. The preparation method disclosed by the invention comprises the steps of purifying industrial fluorene of which the purity is more than or equal to 95% at first, and then preparing the 9-fluorenone by using the high-purity fluorene, so that a purification process of the product namely 9-fluorenone which is difficult to purify is avoided, and the preparation process is simplified. Under the condition of the invention, the conversion rate of fluorene can reach 100%, and fluorenone with the purity of 100% can be obtained; after a reaction solution is cooled and crystal fluorenone is separated out, the filtrate containing the alkali, quaternary ammonium salt, fluorene and fluorenone is directly recycled, and a high-boiling-point solvent is used for absorbing the solvent in the reaction tail gas, so that the method is an energy-saving and environment-friendly chemical process.
Owner:BAOSHUN TECH CO LTD +1
Compound with 9-fluorenone as nucleus and application of same to OLED device
ActiveCN106467484ADestroy crystallinityInhibit aggregationOrganic chemistrySolid-state devicesLight-emitting diodeOLED
The invention discloses a compound with 9-fluorenone as a nucleus and application of the same to an OLED device. The compound uses 9-fluorenone as the nucleus and has the characteristics that molecules are not prone to crystallization and aggregation and the compound has good film forming ability. Applied as a luminescent layer material to an organic light-emitting diode, the compound has good photoelectric performance and can better adapt to and meet application requirements of panel manufacturers.
Owner:JIANGSU SUNERA TECH CO LTD
Bisphenol fluorene synthesizing process catalyzed with solid supported heteropolyacid
InactiveCN1986509AEasy to recycleThe synthesis process is reasonable and feasibleCatalyst carriersOrganic chemistryHeteropoly acidCarboxylic acid
The bisphenol fluorine synthesizing process includes mixing phenol, 9-fluorenone, solid supported heteropoly acid and mercapto carboxylic acid, with phenol / 9-fluorenone molar ratio of 6-14 to 1, solid supported heteropoly acid accounting for 5.0-20.0 % of total reactant weight, heteropoly acid / solid carrier weight ratio of 0.3-1 to 1 and mercapto carboxylic acid / 9-fluorenone weight ratio of 0.02-0.50 to 1, to react at 60-130 deg.c via stirring for 6-14 hr; filtering the reacted product while it is still hot, and recovering the catalyst; washing the catalyst, merging the washed liquid with the filtrate, crystallizing, filtering, drying, recrystallizing in organic solvent and vacuum drying to obtain bisphenol fluorine crystal. The present invention has product purity as high as 99.8 %, simple post-treatment, less metal corrosion and environmental pollution and recovery of catalyst, and may be used widely in the phenol and 9-fluorenone condensation reaction.
Owner:HARBIN ENG UNIV
Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene
ActiveCN104030899AEasy to recycleRaw materials are cheap and easy to getOrganic chemistryOrganic compound preparationEthylene glycol monophenyl etherChemical synthesis
The invention discloses a method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene and belongs to the technical field of chemical synthesis. The method is characterized by comprising the following steps: reacting 9-fluorenone and ethylene glycol monophenyl ether in hydrogen fluoride in the presence of a promoter to obtain 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene; at the end of the reaction, evaporating to reclaim hydrogen fluoride from the reaction system, diluting residues with alkaline liquid; extracting, washing and re-crystallizing to obtain 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene. The method has the advantages of use of low-price and easily available raw materials, high reaction yield, convenience in acid catalyst reclamation, generation of less 'three waste', environmental friendliness and the like, and has a high industrial application value.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Ultraviolet light excited three-component white light fluorescent powder and preparation method thereof
InactiveCN106928996AStable LuminescenceGood thermal stabilityChemical industryEnergy efficient lightingRare earth ionsUltraviolet lights
The invention discloses ultraviolet light excited three-component white light fluorescent powder. The ultraviolet light excited three-component white light fluorescent powder is formed by mixing blue fluorescent powder, green fluorescent powder and red fluorescent powder according to the mass percent of 30 to 40 percent, 25 to 35 percent and 30 to 35 percent, wherein the blue fluorescent powder is graphite phase carbon nitride treated by nitric acid; the green fluorescent powder is carbon nitride modified by a phenyl group; the red fluorescent powder is 2,7 dibromo(4-(diphenyl aminophenyl)phenyl)-9-fluorenone. The invention also discloses a preparation method of the ultraviolet light excited three-component white light fluorescent powder. The three-component white light fluorescent powder disclosed by the invention does not contain rare earth ions and can be matched with an ultraviolet light emitting diode to generate white light; the ultraviolet light excited three-component white light fluorescent powder has the advantages of lower preparation temperature, energy saving, environment friendliness and simple process.
Owner:SOUTH CHINA UNIV OF TECH
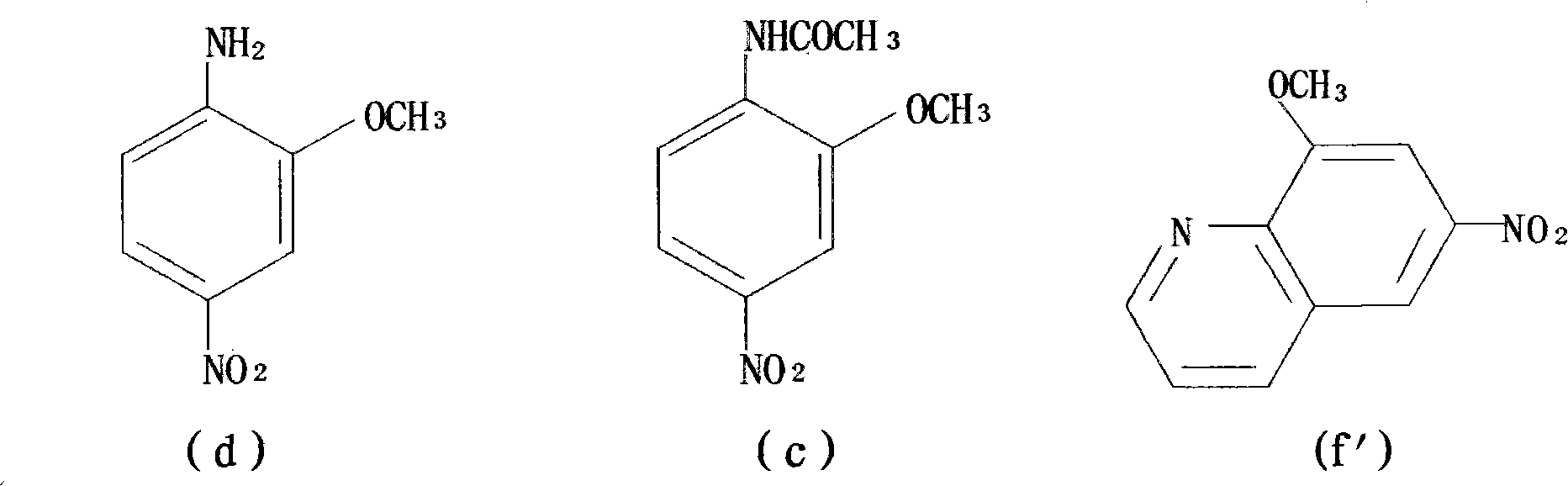
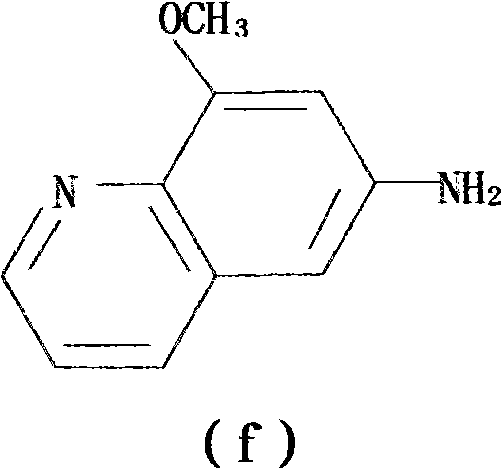
Preparation method of 1,8-dinitro-9-fluorenone
The invention discloses a preparation method of 1,8-dinitro-9-fluorenone (DFO). The preparation method of DFO comprises the following steps that 2-methoxy-4-nitroaniline is synthesized from 2-methoxyaniline; the 2-methoxy-4-nitroaniline is reduced into 2-methoxy-p-phenylenediamine sulfate; the 2-methoxy-p-phenylenediamine sulfate undergoes Skraup quinoline synthesis twice to produce 5(6)-methoxy-4,7-naphthisodiazine; the 5(6)-methoxy-4,7-naphthisodiazine undergoes an oxidation reaction to produce 4,7-naphthisodiazine-5,6-diketone; and the 4,7-naphthisodiazine-5,6-diketone is circulated in an alkaline aqueous solution to produce a product DFO, wherein an overall yield of the five DFO synthesis steps is 26.3%. The preparation method of DFO simplifies a preparation process and effectively reduces a production cost. Through the preparation method of DFO, DFO output can satisfy domestic demands on DFO.
Owner:SUZHOU BEC BIOLOGICAL TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
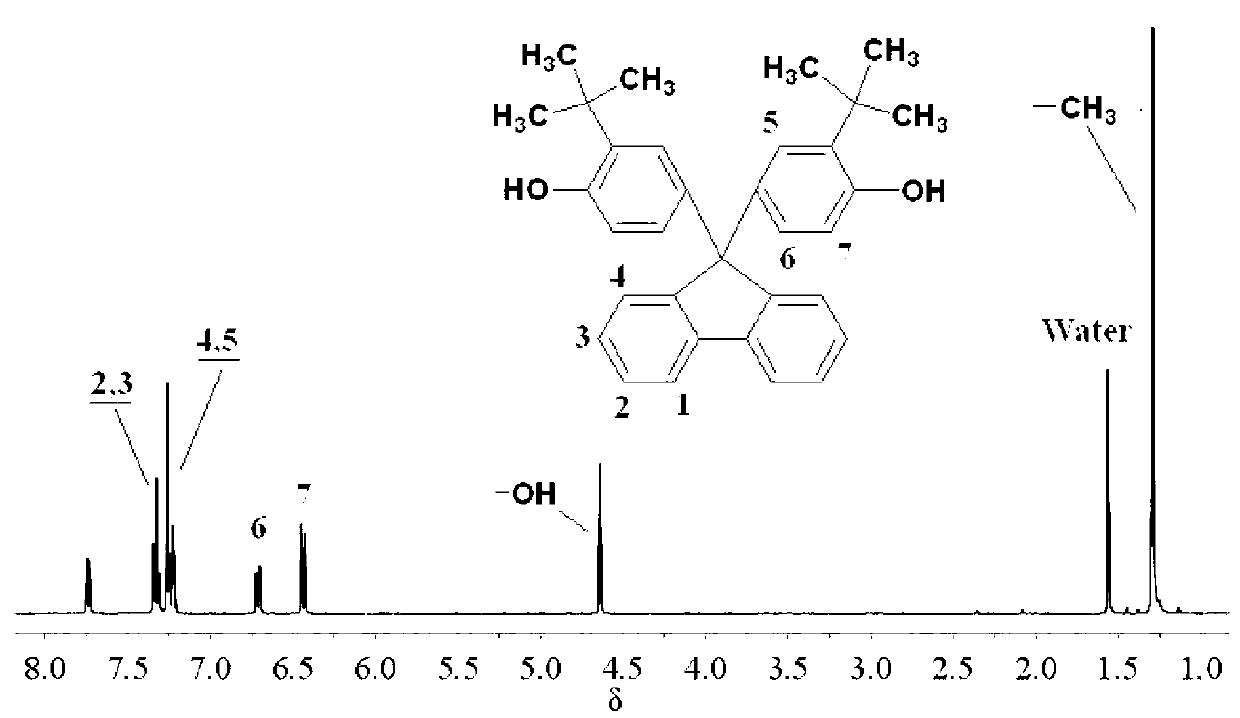
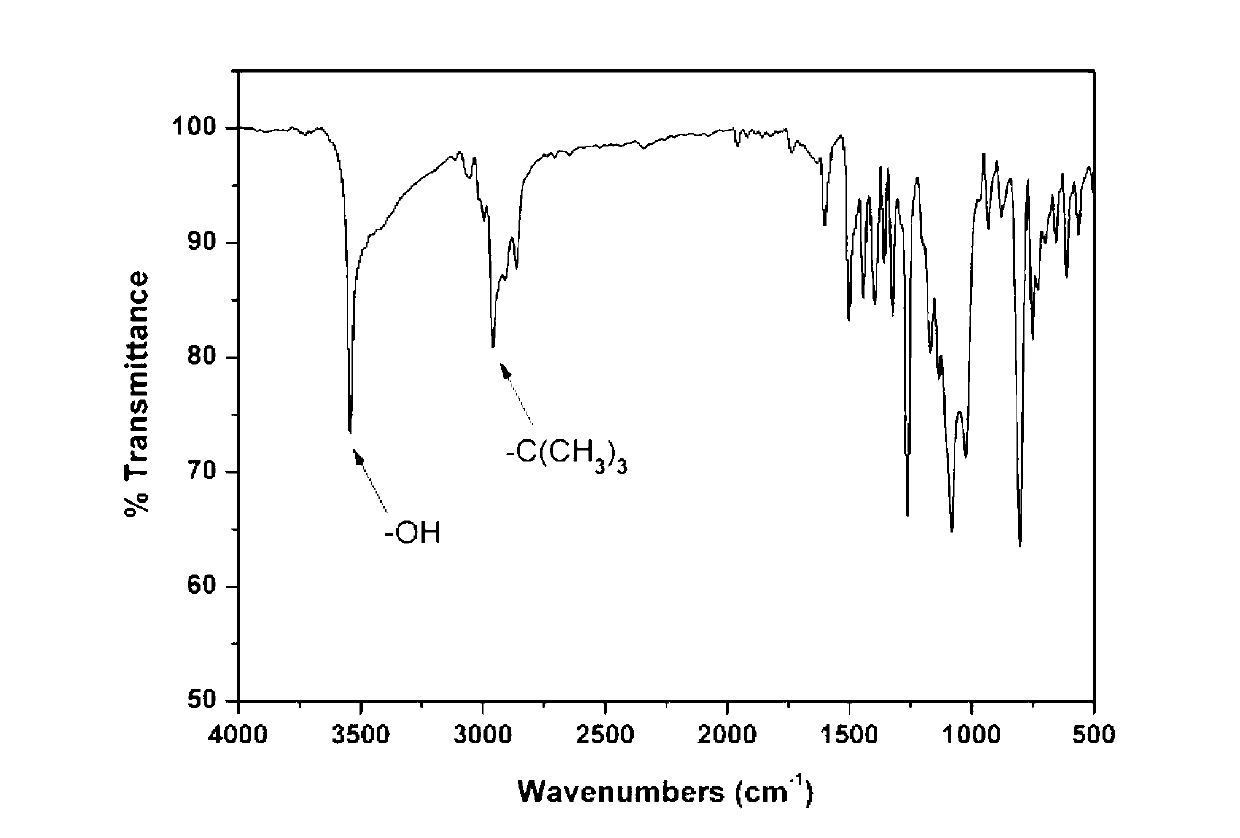
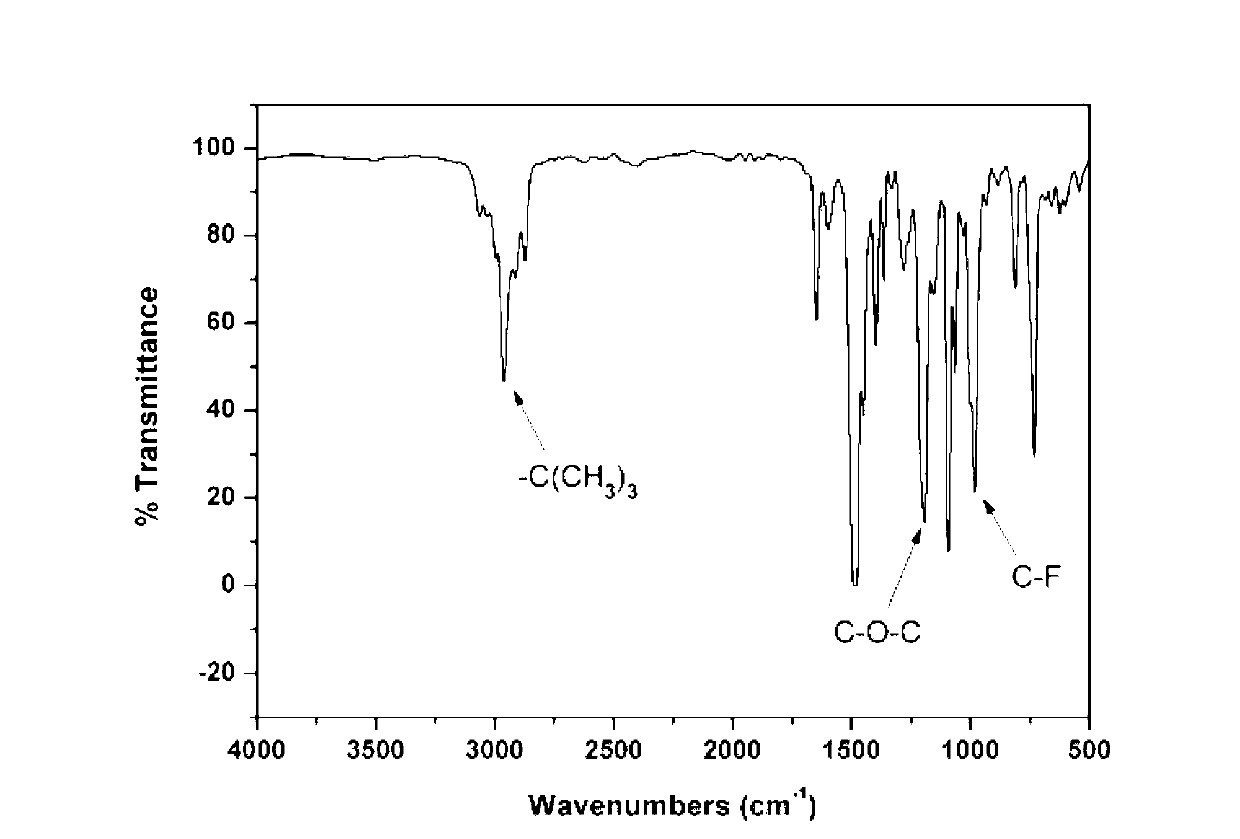
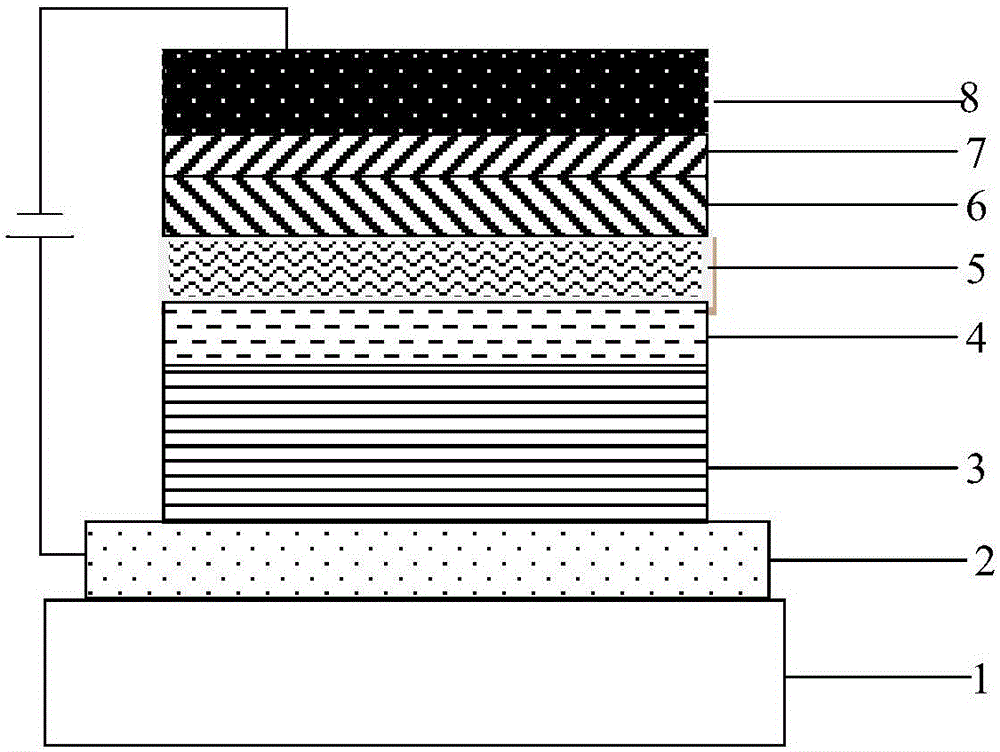
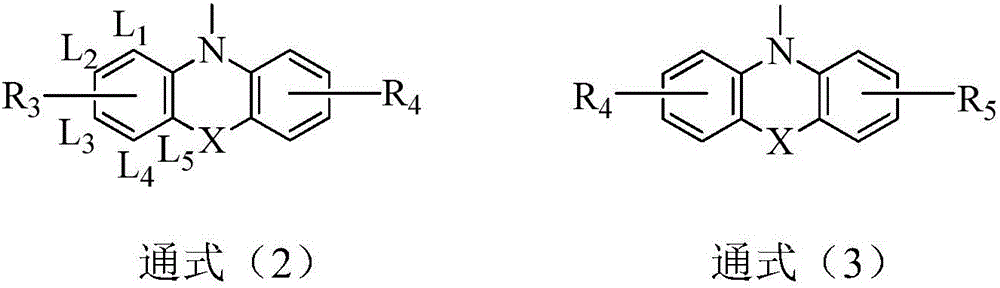

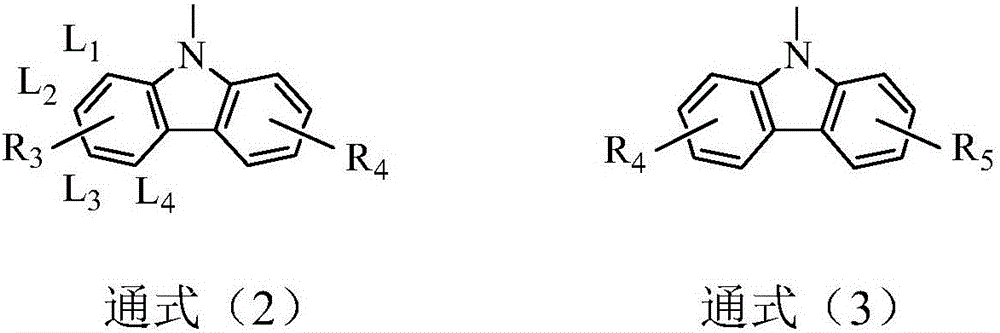
![Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene](https://images-eureka.patsnap.com/patent_img/086a5d27-4106-4340-a6a4-0927f2a5be7f/BDA0000516977770000011.PNG)
![Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene](https://images-eureka.patsnap.com/patent_img/086a5d27-4106-4340-a6a4-0927f2a5be7f/BDA0000516977770000012.PNG)
![Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene Method for preparing 9,9-bis[(4-hydroxy oxyethyl) phenyl] fluorene](https://images-eureka.patsnap.com/patent_img/086a5d27-4106-4340-a6a4-0927f2a5be7f/BDA0000516977770000021.PNG)