Preparation method and application of Schiff base derivative based on tricarbazole
A technology of trioxacarbazole and its derivatives, which is applied in the field of detection, can solve the problems of complex material preparation and difficult detection of HCl gas, and achieve the effects of simple preparation method, convenient and quick operation, high selectivity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
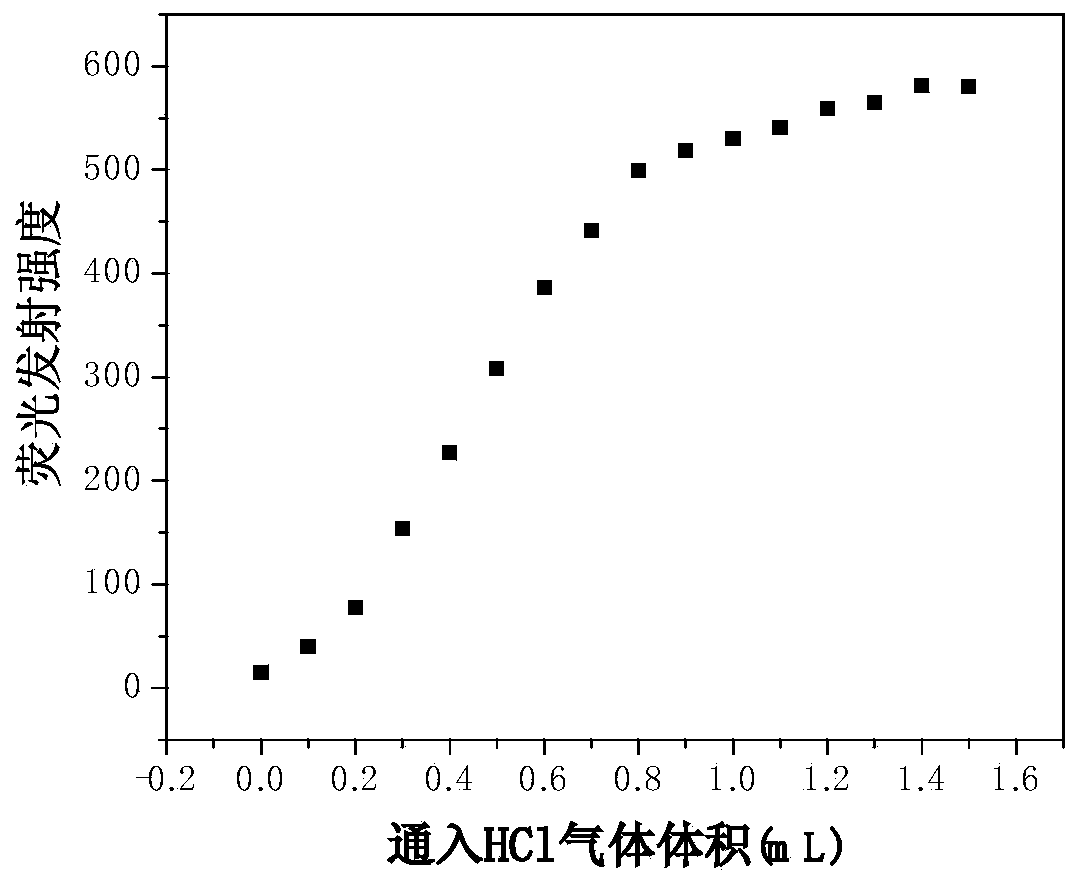
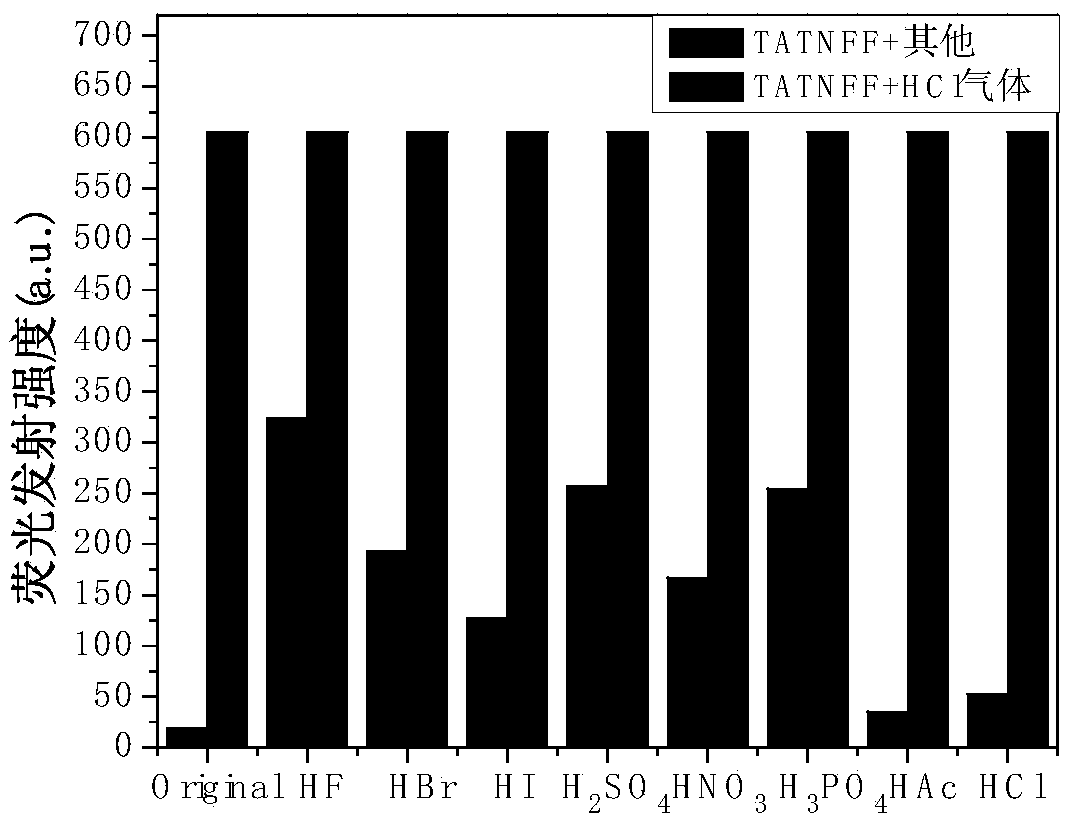
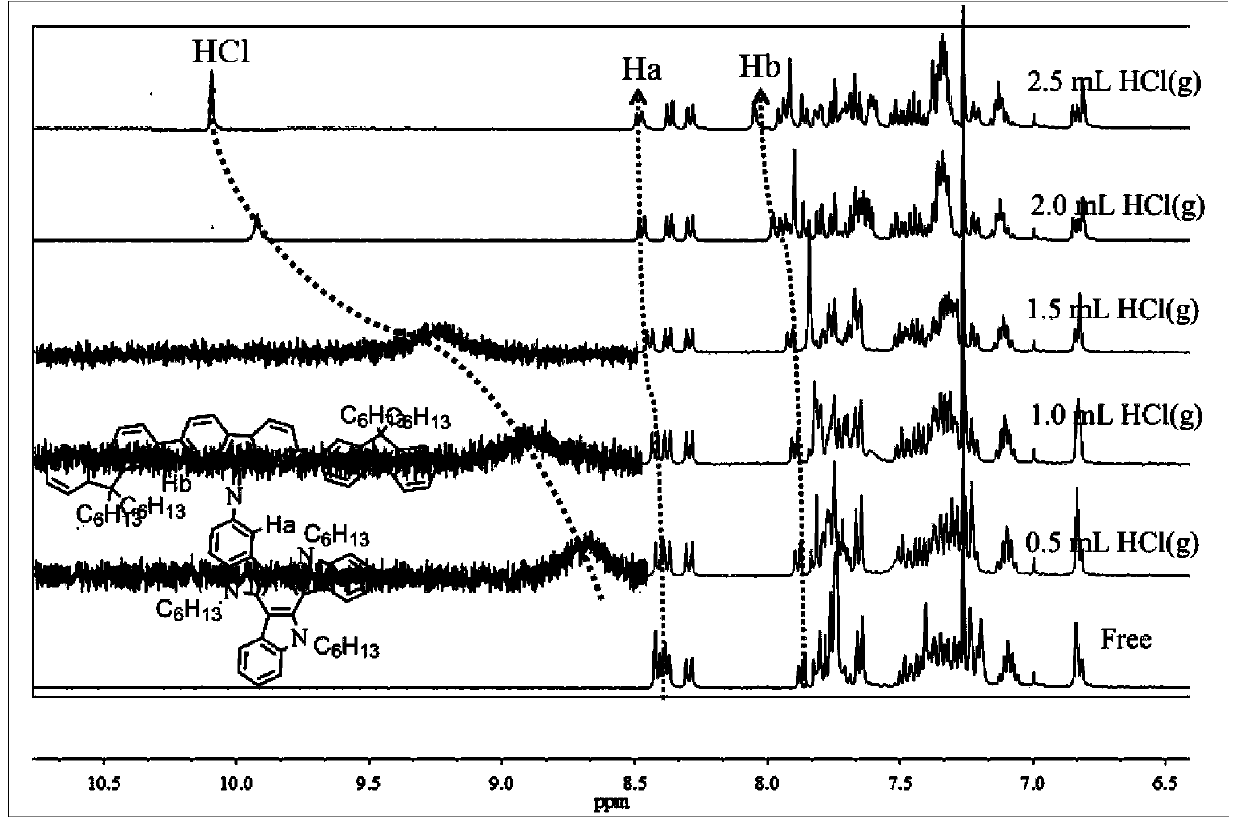
[0031] The preparation method includes: reduction of 3-nitrotrioxcarbazole to 3-amino-trioxcarbazole, and then reacting with 2,7-dibromo-9-fluorenone to synthesize compound II, and finally compound II with different fluorescent light The chromophore Ar undergoes Suzuki reaction to prepare compound I. Such Schiff base derivatives have high sensitivity and good stability for HCl gas detection.
[0032]
[0033] Wherein Ar in formula I is one of the following structures:
[0034]
[0035] Among them, R, R 1 , R 2 And R 3 C1-C12 linear or branched alkyl or alkoxy; * is the position of attachment; N is a nitrogen atom; S is a sulfur atom.
[0036] The preparation method of the ternary carbazole Schiff base derivative material includes the following steps:
[0037] Step 1: Preparation of 3-amino-trioxcarbazole: Under nitrogen protection, dissolve 1mmol of 3-nitro-trioxcarbazole and 0.6-1.0mmol of palladium on carbon (Pd / C) catalyst in 5-10mL N,N-dimethylformamide (DMF), slowly add 8-12mmo...
Embodiment 1
[0046]
[0047] Reaction condition 1: Under nitrogen protection, combine 3-nitro-5,10,15-trihexyl-triscarbazole (643.0mg, 1.0mmol) and Pd / C (9.8mg, 0.6mmol) with 5mL of N ,N-Dimethylformamide (DMF) is mixed, and the temperature is controlled at 45℃, slowly adding N 2 H 4 ·H 2 O (0.4mL, 8.0mmol), react for 8h. After the reaction, it was cooled to room temperature, extracted with dichloromethane, then dried with anhydrous magnesium sulfate, filtered, and the solvent was evaporated to separate and purify the solid obtained by column chromatography to obtain a red powder (459 mg) with a yield of 74.8%.
[0048] Reaction condition 2: Under nitrogen protection, combine 3-nitro-5,10,15-trihexyl-triscarbazole (643.0mg, 1.0mmol) and Pd / C (13.0mg, 0.8mmol) with 8mL of DMF Mix and slowly add N under the condition of temperature control 55℃ 2 H 4 ·H 2 O (0.5 mL, 10.0 mmol), react for 10 h. After the reaction, it was cooled to room temperature, extracted with dichloromethane, dried with anhy...
Embodiment 2
[0052]
[0053] Reaction condition 1: Add 3-amino-5,10,15-trihexyl-trioxcarbazole (613.0mg, 1.0mmol) and 2,7-dibromo-9-fluorenone (338.0mg, 1.0mmol) to Add 15 mL of ethanol and 0.15 mL of glacial acetic acid to the two-necked flask, and reflux for 5 hours at 100°C. After the reaction, it was cooled and filtered with suction, washed with a small amount of anhydrous ethanol, dried, and recrystallized with anhydrous ethanol to obtain a dark red solid compound II (643 mg) after drying, with a yield of 69.0%.
[0054] Reaction condition 2: Add 3-amino-5,10,15-trihexyl-triscarbazole (613.0mg, 1.0mmol) and 2,7-dibromo-9-fluorenone (338.0mg, 1.0mmol) to Add 20 mL of ethanol and 0.2 mL of glacial acetic acid to the two-neck flask, and reflux for 6 hours at 110°C. After the reaction, it was cooled and filtered with suction, washed with a small amount of anhydrous ethanol, dried, and recrystallized with anhydrous ethanol to obtain a dark red solid compound II (695 mg) after drying, with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com