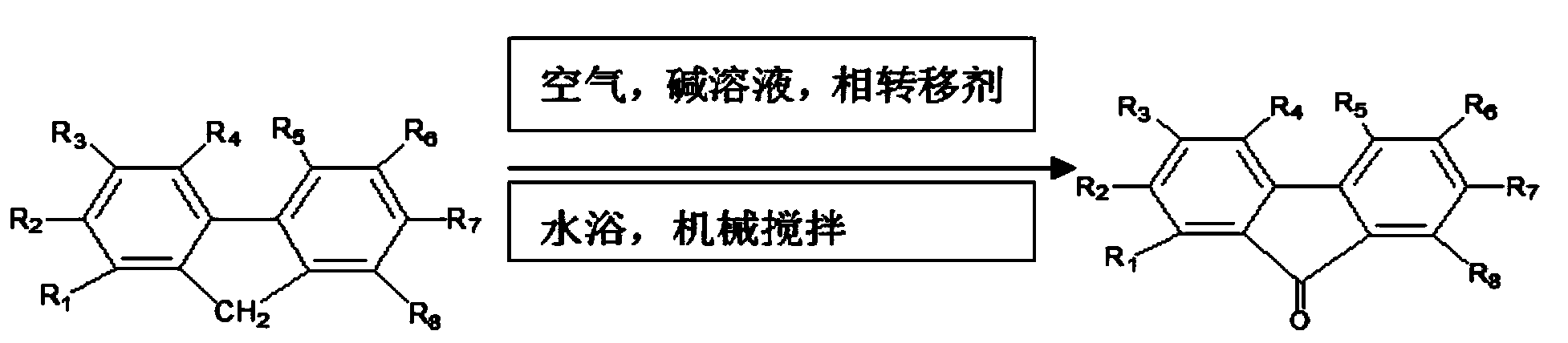
Preparation method for 9-fluorenone
The technology of fluorenone and quaternary ammonium salt is applied in the field of preparation of 9-fluorenone, which can solve the problems of human health damage, pyridine odor, environmental pollution and the like, and achieve the effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] ① Take 5g of industrial fluorene, 0.1g of reagent grade tetra-n-butylammonium bromide and 20mL of quinoline, put them in a 50mL three-neck round bottom flask, and mechanically stir in a constant temperature water bath at 60°C until completely dissolved to obtain an oil phase;
[0046] ② Dissolve sodium hydroxide in 4mL water to prepare a 30wt% aqueous phase;
[0047] ③Mix the oil phase and the water phase, stir mechanically in a constant temperature water bath at 60°C at a stirring speed of 300r / min, feed air at a speed of 140mL / min, monitor the reaction process with thin-layer chromatography, after 2.5h, thin-layer The chromatogram showed that the fluorene spot of the raw material disappeared completely, and the reaction was stopped after continuing the reaction for 0.5h to obtain the reaction solution a;
[0048] ④ Cool and stratify the reaction liquid a, separate the water layer, recover the alkaline solution, take the oil layer, wash the oil layer with water until it ...
Embodiment 2
[0051] Except that in step 2, 4 mL of 40 wt % sodium hydroxide solution was used instead of 4 mL of 30 wt % sodium hydroxide solution, and steps 1 and 3 were changed to stirring, dissolving and reacting at 40 ° C, the others were the same as in Example 1. After 9.5 hours of reaction, thin-layer chromatography showed that the raw material fluorene spots completely disappeared, and the reaction was continued for 0.5 hours to stop the reaction. Obtain 5.0g product after step ④ a series of processing.
[0052] Samples were taken for GC analysis, and the spectrum showed that there was no fluorenone component. Except for the impurities brought in by 9-fluorenone and raw material industrial fluorene, no new components appeared, which also indicated that fluorene was 100% converted into 9-fluorenone.
Embodiment 3
[0054] Except that in step ②, 4mL of 40wt% sodium hydroxide solution was used instead of 4mL of 30wt% sodium hydroxide solution, and steps ① and ③ were changed to stirring, dissolving and reacting at 50°C, the others were the same as in Example 1. After 6.5 hours of reaction, thin-layer chromatography showed that the raw material fluorene spots completely disappeared, and the reaction was continued for 0.5 hours to stop the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com