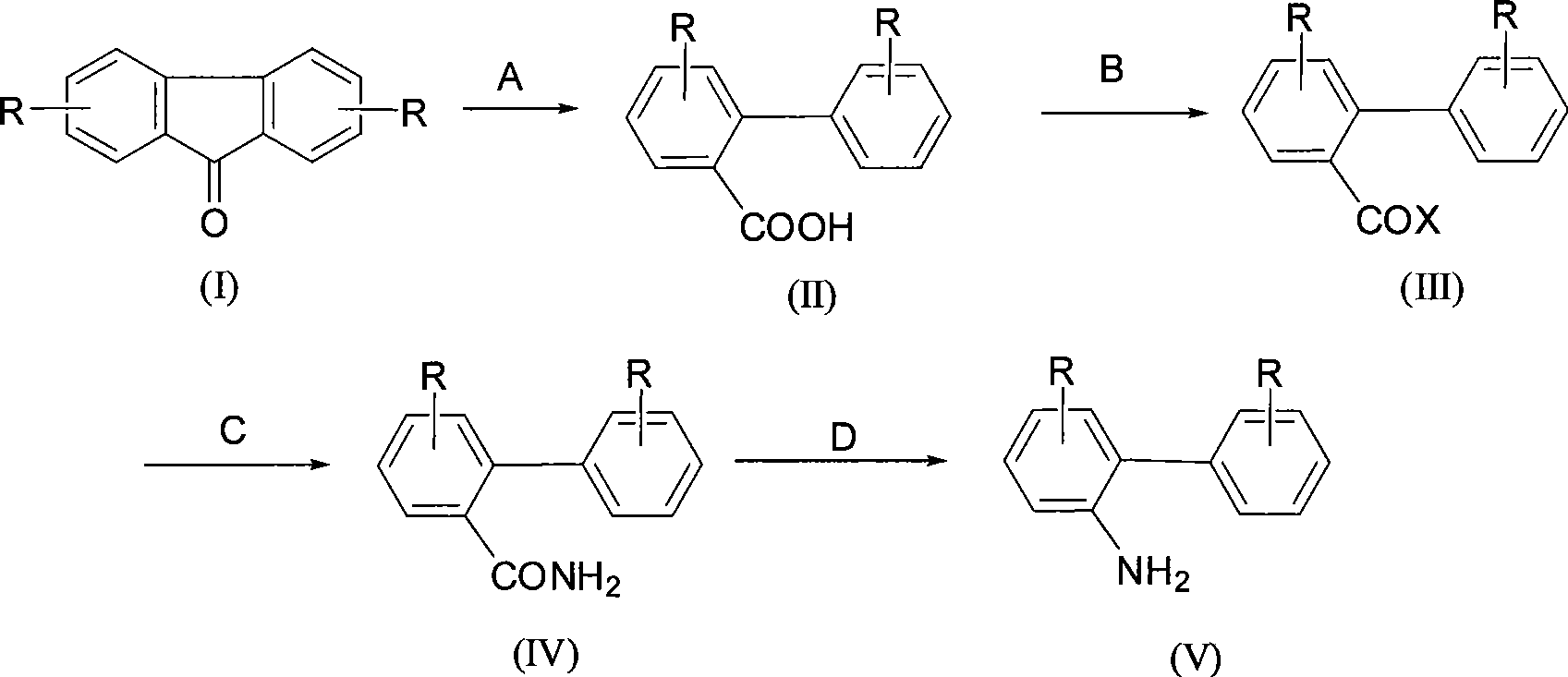
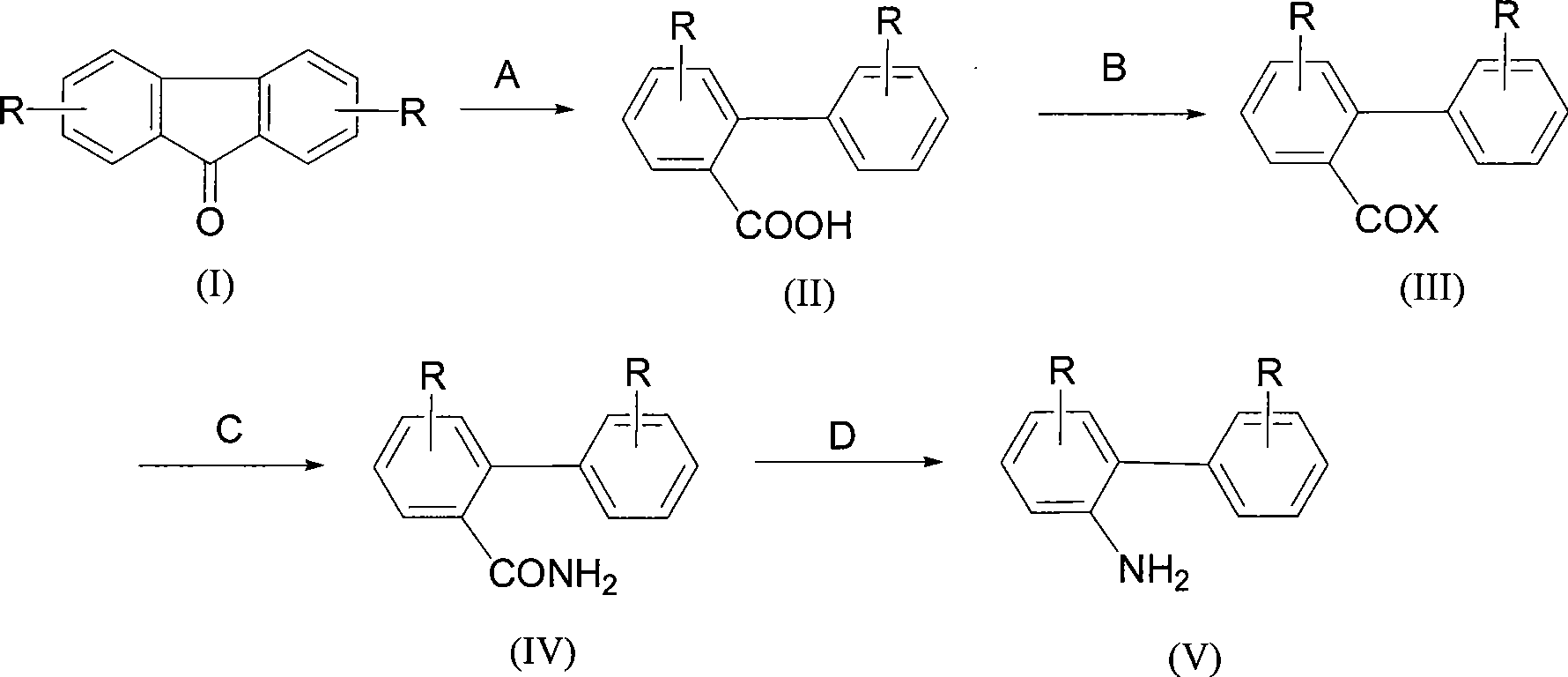
Synthesis of 2-aminobiphenyl compounds
A synthetic method, the technology of aminobiphenyl, applied in the direction of amino compound preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problems of high price, high industrialization cost, high equipment requirements, etc., and achieve low production cost and easy operation , the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 2-biphenylcarboxylic acid
[0036] Add 50g of 9-fluorenone and 400mL of diphenyl ether into a 1L three-necked flask equipped with mechanical stirring, reflux condenser and thermometer, stir and dissolve, add 130g of potassium hydroxide at the same time, heat and reflux for 6 hours, and the reaction is complete. Cool down, acidify with 36-37% hydrochloric acid to pH=1-2, filter with suction, and dry to obtain 2-biphenylcarboxylic acid, the content is ≥99% by HPLC analysis, and the yield is 98%.
Embodiment 2
[0037] The synthesis of embodiment 2 2-biphenylformic acid
[0038] Add 50g of 9-fluorenone and 400mL of toluene into a 1L three-necked flask equipped with mechanical stirring, reflux condenser and thermometer, stir to dissolve, add 130g of potassium hydroxide at the same time, heat and reflux for 6 hours, and the reaction ends. Cool down, acidify with 36-37% hydrochloric acid to pH=1-2, filter with suction, and dry to obtain 2-biphenylcarboxylic acid, the content is ≥99% by HPLC analysis, and the yield is 98%.
Embodiment 3
[0039]Synthesis of Example 3 2-biphenylcarboxylic acid
[0040] Add 10g of 9-fluorenone and 80mL of diphenyl ether into a 1L three-necked flask equipped with mechanical stirring, reflux condenser and thermometer, stir to dissolve, add 20g of potassium tert-butoxide at the same time, heat and reflux for 8 hours, and the reaction is completed. Cool down, acidify with 36-37% hydrochloric acid to pH = 1-2, filter with suction, and dry to obtain 2-biphenylcarboxylic acid, the content is ≥99% by HPLC analysis, and the yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com