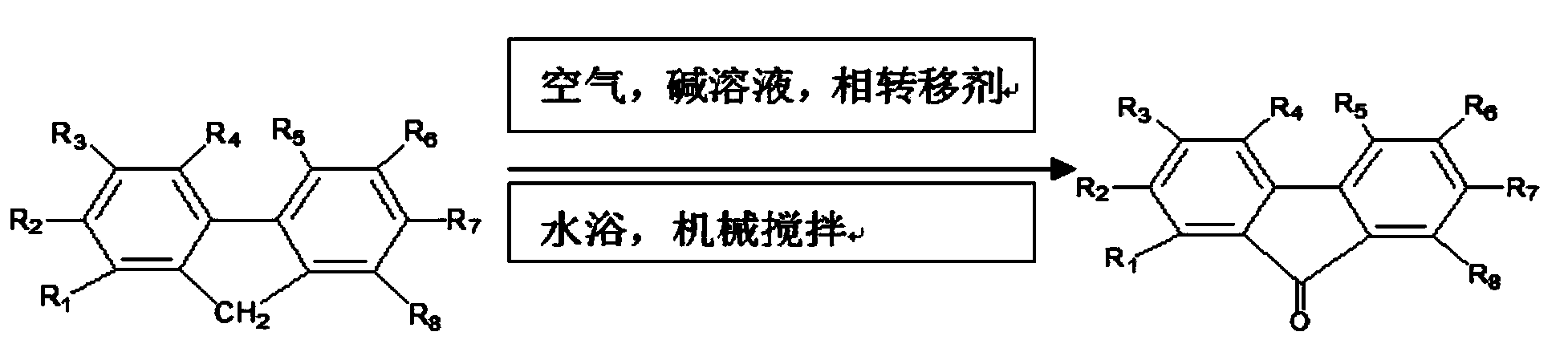
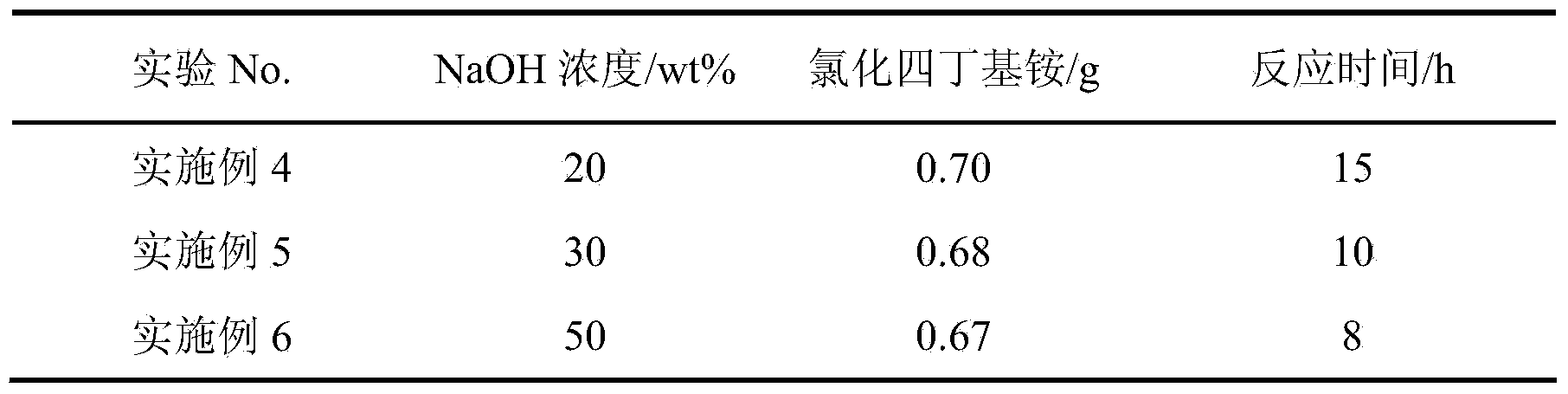
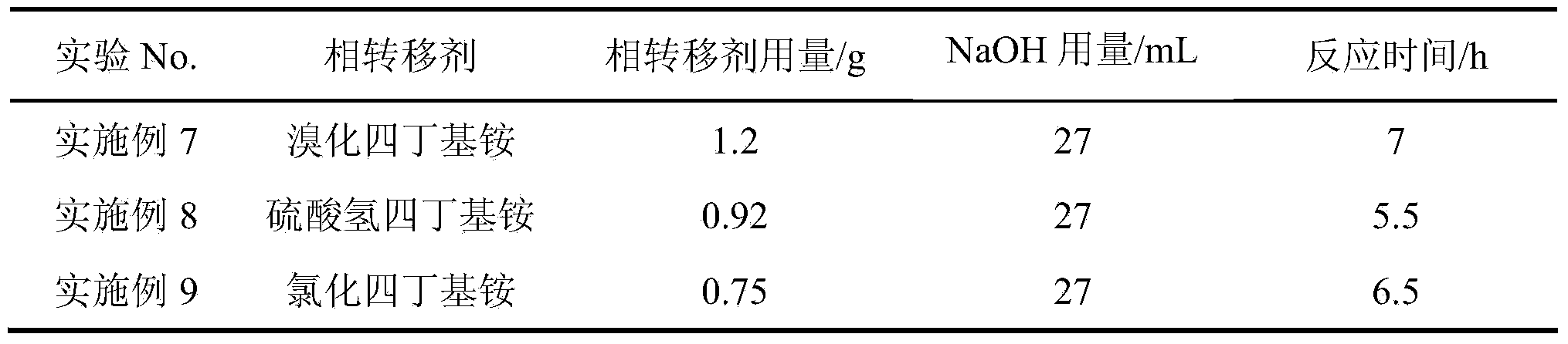
Method for preparing 9-fluorenone via four-phase transfer catalysis
A technology of phase transfer catalysis and fluorenone, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of large solvent loss, difficult phase transfer catalyst effect of quaternary ammonium salts, and high reaction temperature. achieve the effect of improving utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Take 40g of industrial fluorene and place it in a 250mL four-necked flask equipped with mechanical stirring, reflux condenser, and two inlet pipes, add 45mL of xylene, 0.72g of tetrabutylammonium chloride and 9mL of 40wt% NaOH solution, and place in a water bath at 40°C Heating at medium temperature, controlling the stirring rate to 300r / min, feeding 200*2mL / min air, and connecting the outlet of the reflux condenser tube to the inlet of the tail gas absorption tube equipped with α-methylnaphthalene. At the beginning of the reaction, fluorene was suspended. The reaction process was monitored by thin chromatography, and the raw material fluorene spots disappeared after 6 hours of reaction, and the reaction was continued for 0.5 hours to stop the reaction. The reaction liquid was transferred to a 500mL beaker while it was hot, and pale yellow crystals were precipitated immediately. After no more crystals were precipitated, vacuum filtration was performed to obtain crystal ...
Embodiment 2
[0058] Except that raw material fluorene is increased to 80g, and tetrabutylammonium chloride is increased to 1.7g, other is the same as embodiment 1. After 8 hours of reaction, TLC showed that the raw material fluorene spots completely disappeared, and the reaction was continued for 0.6 hours to stop the reaction.
[0059] 64.08 g of crystal i was obtained, and the content of 9-fluorenone was >99.5% according to chromatographic analysis;
[0060] Crystal II weighed 15.1 g, and the 9-fluorenone content was 97% according to chromatographic analysis.
Embodiment 3
[0062] Except that raw material fluorene is increased to 60g, other is the same as embodiment 1. After 7 hours of reaction, thin-layer chromatography showed that the raw material fluorene spots completely disappeared, and the reaction was continued for 0.5 hours to stop the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com