Dual-substituent-group-9-fluorenone compound-containing organic light-emitting device and application thereof
A technology of electroluminescent devices and compounds, applied in the field of organic electroluminescent devices, can solve the problems of high exciton utilization rate, high fluorescence radiation efficiency, efficiency roll-off, low S1 state radiation transition rate, etc., and achieve high efficiency , the effect of small energy gap difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
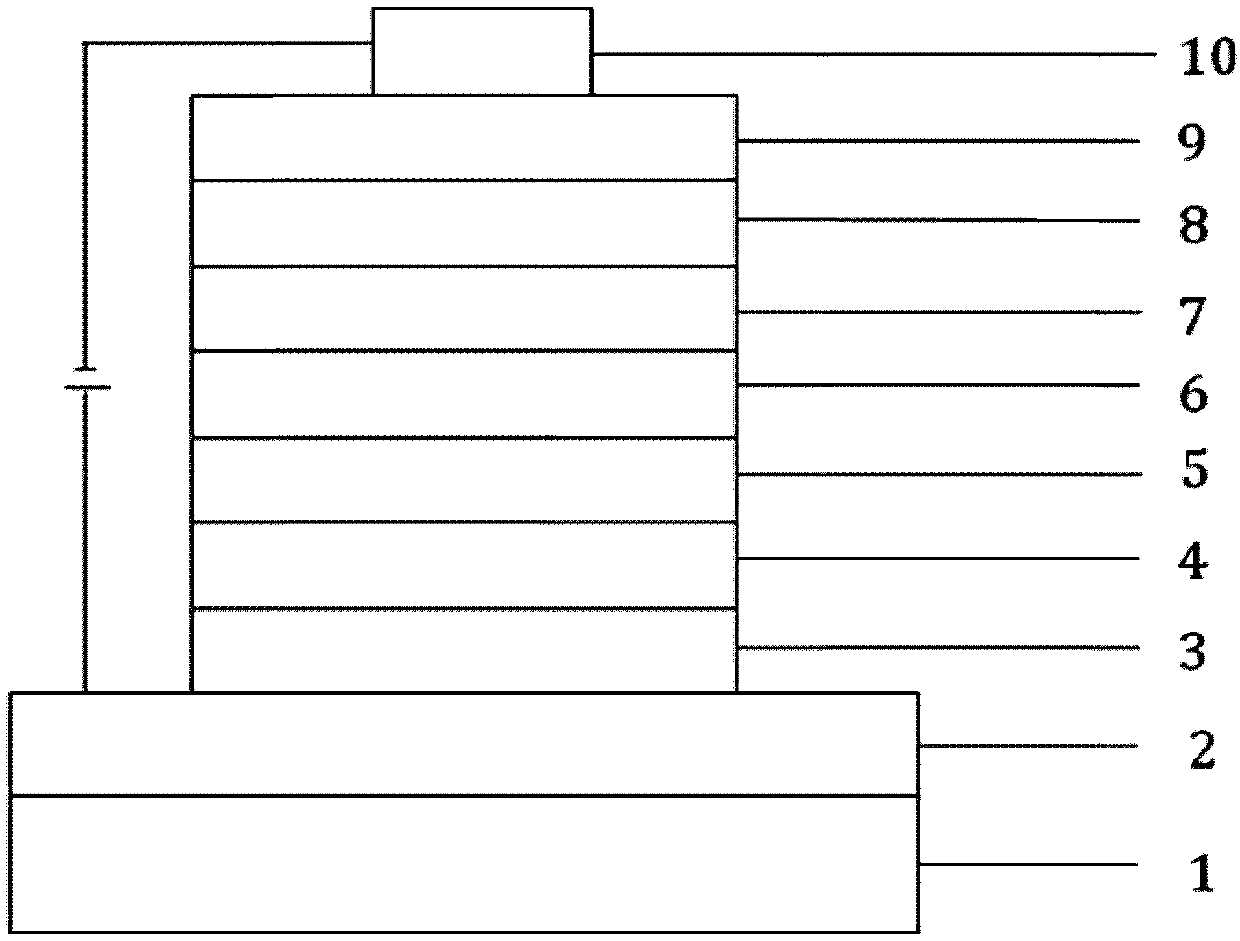
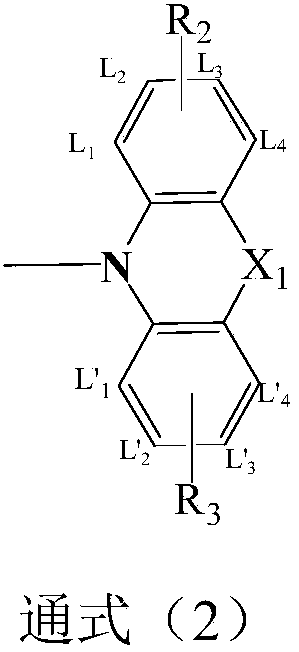
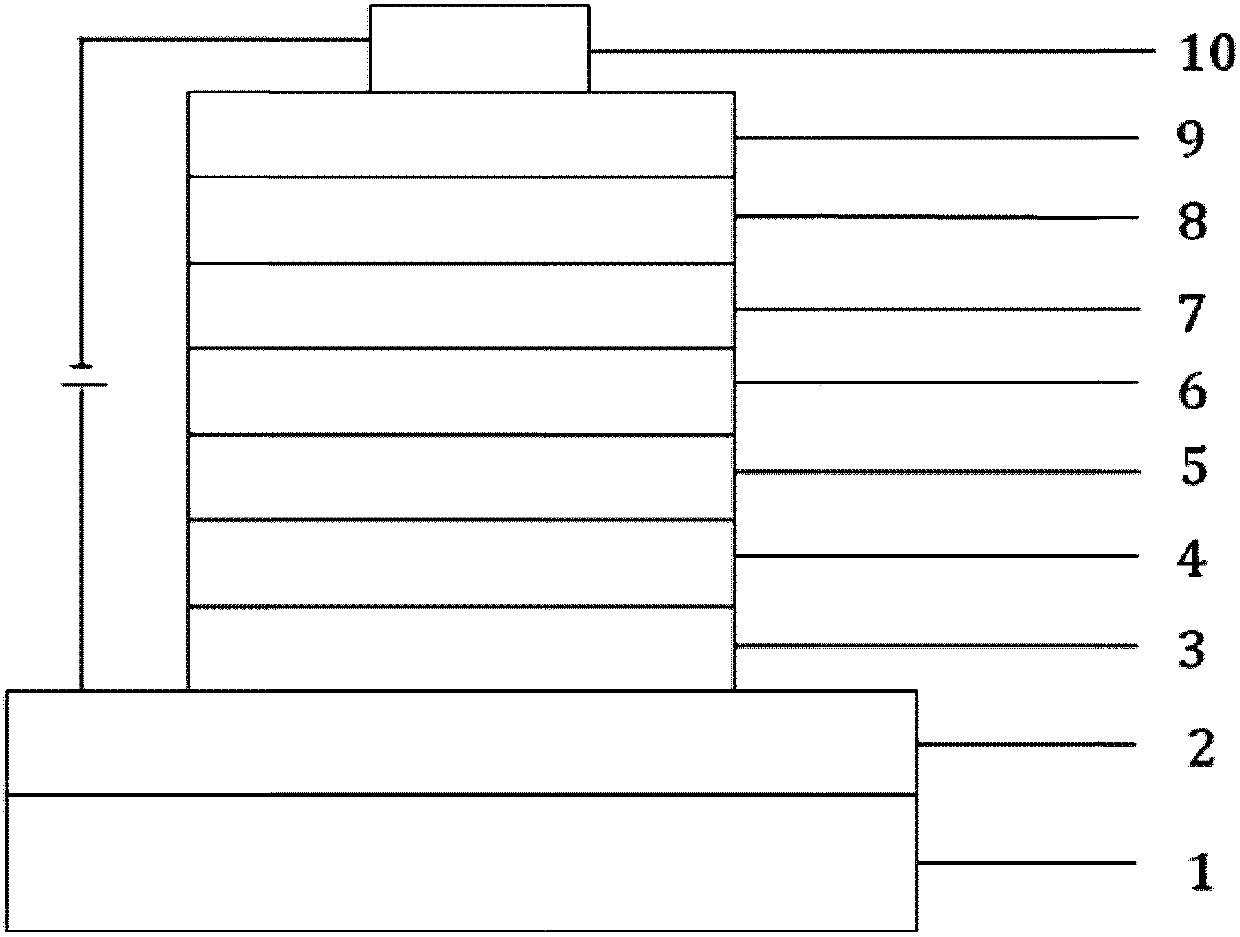
Image
Examples
Embodiment 1
[0059] Example 1 Compound 1
[0060]
[0061] In a 250mL three-neck flask, under nitrogen atmosphere, add 0.01mol (3.38g) 2,7-dibromo-9-fluorenone, 0.025mol (4.58g) compound M1, 0.03mol (2.88g) tert-butanol Sodium, 10 -4 mol(0.073g)Pd(dppf)Cl 2 , 180mL toluene, heated to reflux for 8 hours, sampled and plated, the raw materials were completely reacted; naturally cooled to room temperature (20-25°C), filtered, and the filtrate was collected for vacuum rotary evaporation (-0.09MPa, 85°C), and column chromatography , the target product was obtained with a purity of 99% and a yield of 87%.
[0062] Elemental analysis structure (molecular formula C37H22N2O3): Theoretical value C, 81.90; H, 4.09; N, 5.16; O, 8.85 Test value: C, 81.98; H, 4.05; N, 5.08; O, 8.89.
[0063] HPLC-MS: The theoretical molecular weight of the material is 542.16, and the actual molecular weight is 542.49.
Embodiment 2
[0064] Example 2 Compound 2
[0065]
[0066] In a 250mL three-neck flask, under nitrogen atmosphere, add 0.01mol (3.38g) 2,7-dibromo-9-fluorenone, 0.025mol (5.83g) compound M2, 0.03mol (2.88g) tert-butanol Sodium, 10 -4 mol(0.073g)Pd(dppf)Cl 2 , 180mL toluene, heated to reflux for 10 hours, sampled and spotted, the raw materials were completely reacted; naturally cooled to room temperature (20-25°C), filtered, and the filtrate was collected for vacuum rotary evaporation (-0.09MPa, 85°C), and column chromatography , to obtain the target product with a purity of 98% and a yield of 75%.
[0067] Elemental analysis structure (molecular formula C45H26N2O3): theoretical value C, 84.10; H, 4.08; N, 4.36; O, 7.47 test value: C, 84.13; H, 4.05; N, 4.33;
[0068] HPLC-MS: The theoretical molecular weight of the material is 642.19, and the actual molecular weight is 642.66.
Embodiment 3
[0069] Example 3 Compound 3
[0070]
[0071] In a 250mL three-neck flask, under nitrogen atmosphere, add 0.01mol (3.38g) 2,7-dibromo-9-fluorenone, 0.025mol (5.83g) compound M3, 0.03mol (2.88g) tert-butanol Sodium, 10 -4 mol(0.073g)Pd(dppf)Cl 2 , 180mL of toluene, heated to reflux for 10 hours, sampling point plate, the reaction was complete; naturally cooled, filtered, the filtrate was collected and subjected to vacuum rotary evaporation (-0.09MPa, 85°C), and column chromatography was carried out to obtain the target product with a purity of 99%. Yield 77%.
[0072] Elemental analysis structure (molecular formula C45H26N2O3): theoretical value C, 84.10; H, 4.08; N, 4.36; O, 7.47 test value: C, 84.09; H, 4.07; N, 4.33;
[0073] HPLC-MS: The theoretical molecular weight of the material is 642.19, and the actual molecular weight is 642.68.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com