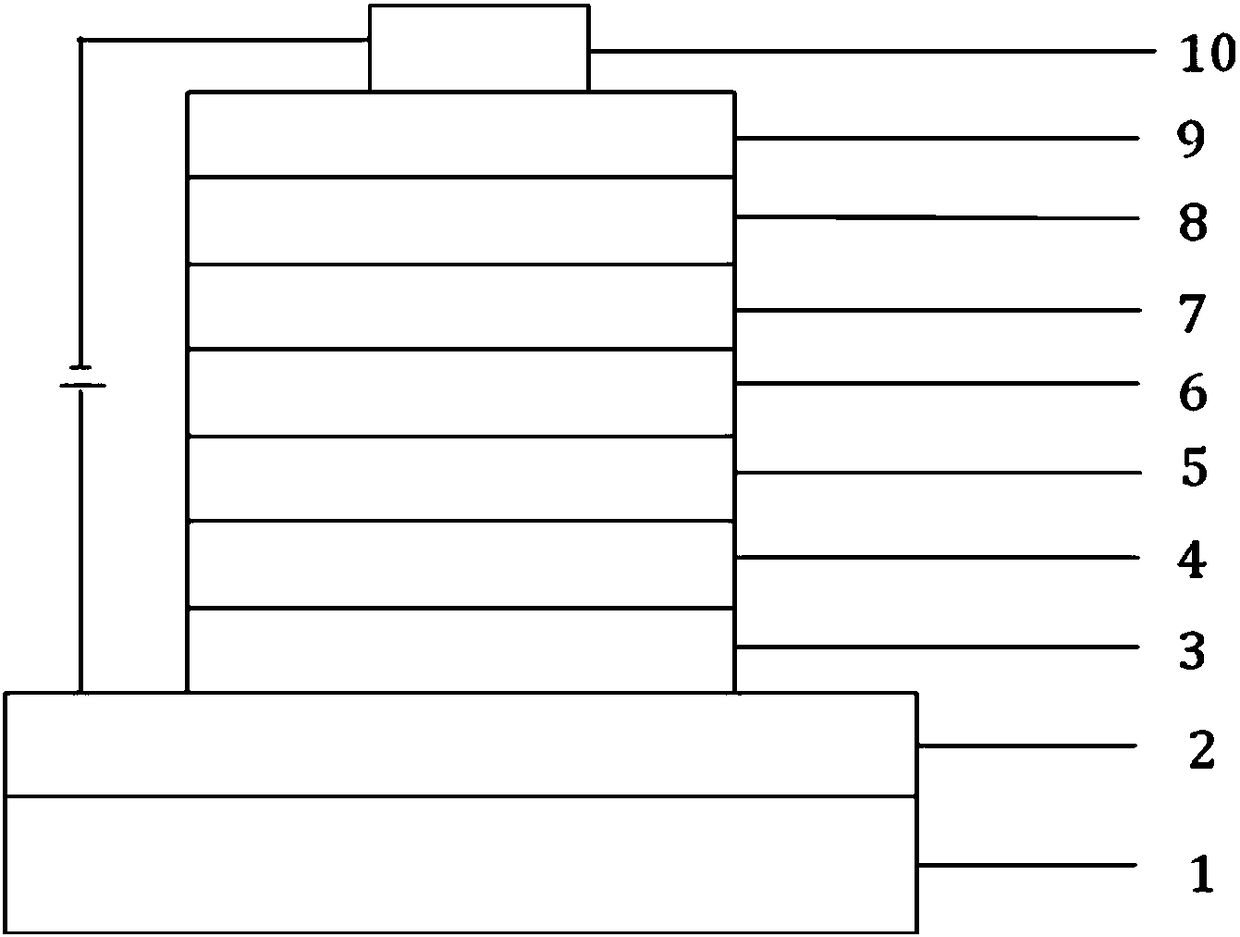
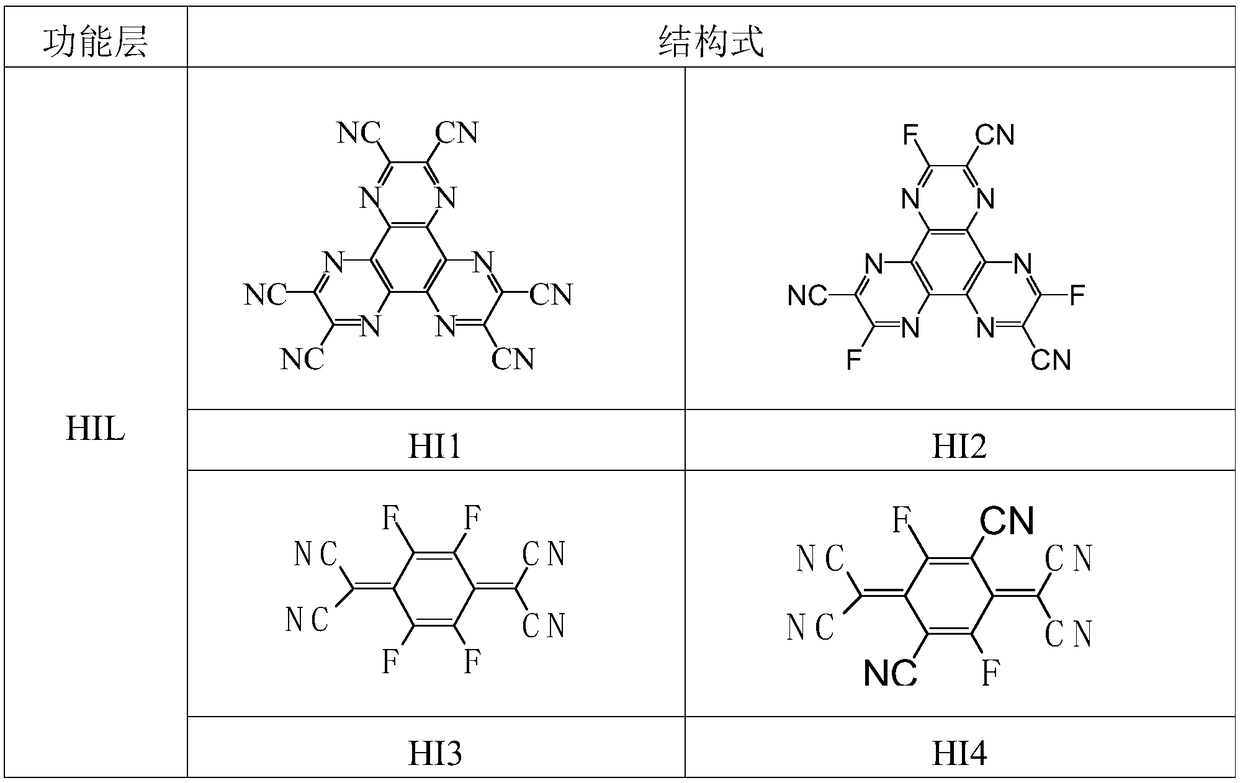
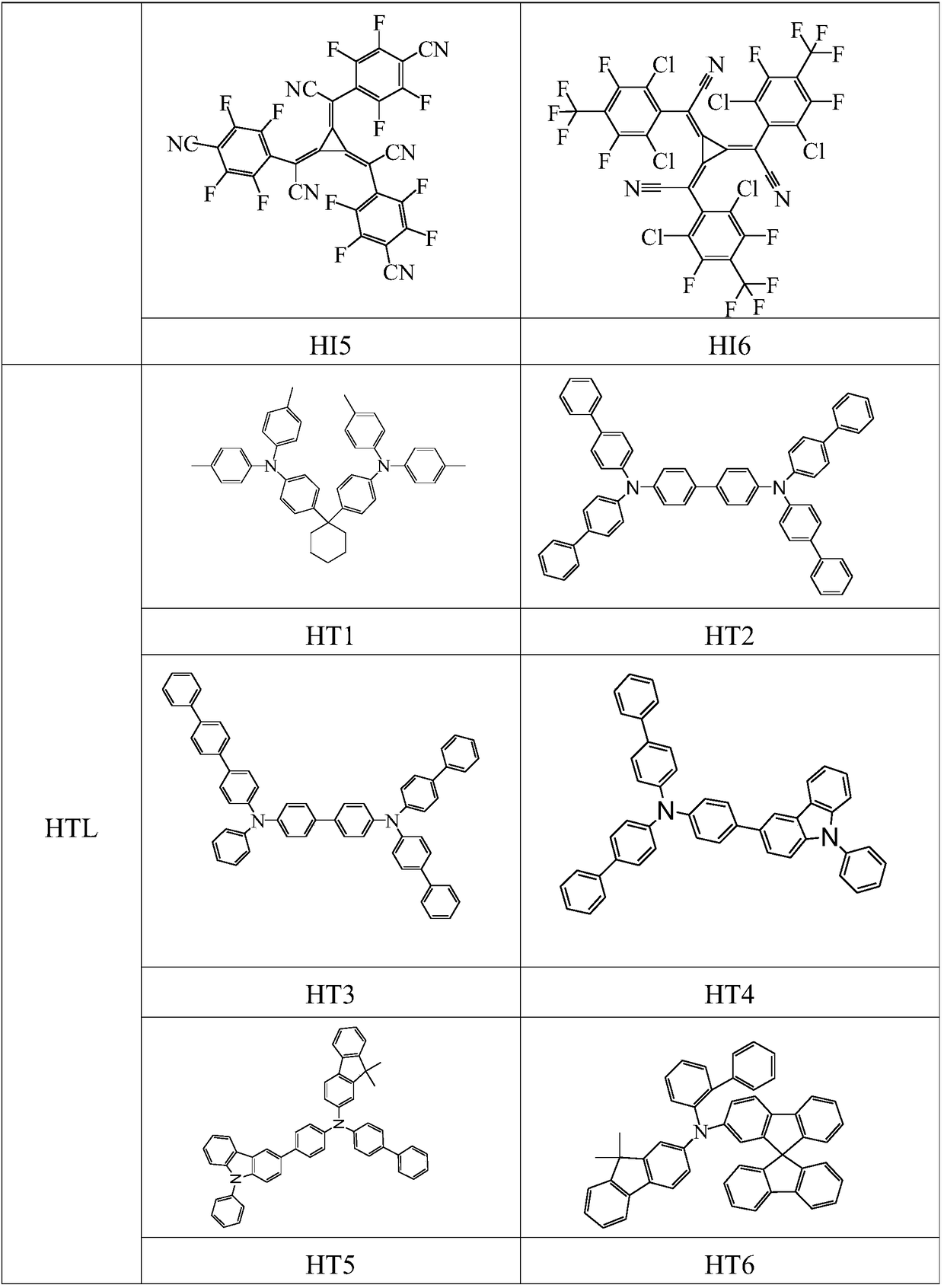
A kind of organic electroluminescent device containing triazine and ketone compound and its application
A technology of electroluminescent devices and ketone compounds, applied in the field of organic electroluminescent devices, can solve problems such as efficiency roll-off, low S1 state radiation transition rate, difficult high exciton utilization rate and high fluorescence radiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Synthesis of Example 1 Compound 1
[0070]
[0071] The concrete synthetic route of this compound is provided now:
[0072]
[0073] In a 500ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4,6-diphenyl-[1,3,5]triazine-2-carbonyl chloride, 0.03mol 9,9-dimethyl- 10-Phenyl-9,10-dihydroacridine 2-boronic acid, dissolved in a mixed solvent (180ml toluene, 90ml ethanol), then added 0.03mol Na 2 CO 3 aqueous solution (2M), then add 0.0001mol Pd (PPh 3 ) 4 , heated to reflux for 10-24 hours, sampled and plated, the reaction was complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a HPLC purity of 99.2% and a yield of 58.00%.
[0074] Elemental analysis structure (molecular formula C 37 h 28 N 4 O): theoretical value C, 81.59; H, 5.18; N, 10.29; O, 2.94; tested value: C, 81.66; H, 5.12; N, 10.25;
[0075] HPLC-MS: The molecular weight of the material is 544.2...
Embodiment 2
[0076] Synthesis of Example 2 Compound 5
[0077]
[0078] The concrete synthetic route of this compound is provided now:
[0079]
[0080] In a 500ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 2-chloro-4,6-bis(3,5-dimethylphenyl)-[1,3,5]triazine, 0.03mol2 , 6-anthraquinone diboronic acid, 0.01mol10-(4-bromophenyl)-9,9-dimethyl-9,10-dihydro-acridine, dissolved in a mixed solvent (180ml toluene, 90ml ethanol), then Add 0.03molNa 2 CO 3 aqueous solution (2M), then add 0.0001mol Pd (PPh 3 ) 4 , heated to reflux for 10-24 hours, sampled and plated, the reaction was complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with an HPLC purity of 99.0% and a yield of 35.00%.
[0081] Elemental analysis structure (molecular formula C 54 h 42 N 4 o 2 ): theoretical value C, 83.26; H, 5.43; N, 7.19; O, 4.11; test value: C, 83.35; H, 5.36; N, 7.22;
[0082] HPLC-MS: The mo...
Embodiment 3
[0083] Synthesis of Example 3 Compound 6
[0084]
[0085] The concrete synthetic route of this compound is provided now:
[0086]
[0087] In a 500ml four-neck flask, add 0.01mol 2-chloro-4-naphthalen-2-yl-6-phenyl-[1,3,5]triazine, 0.03mol 2,7-oxa Anthrone diboronic acid, 0.01mol 2-bromo-9,9,10-triphenyl-9,10-dihydroacridine, dissolved in a mixed solvent (180ml toluene, 90ml ethanol), then added 0.03mol Na 2 CO 3 aqueous solution (2M), then add 0.0001mol Pd (PPh 3 ) 4, heated to reflux for 10-24 hours, sampled and plated, the reaction was complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with an HPLC purity of 98.96% and a yield of 46.00%.
[0088] Elemental analysis structure (molecular formula C 63 h 40 N 4 o 2 ): theoretical value C, 85.50; H, 4.56; N, 6.33; O, 3.62; test value: C, 85.56; H, 4.52; N, 6.31;
[0089] HPLC-MS: The molecular weight of the material is 884...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com