Method for producing 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene, crystal of said compound, and method for producing said crystal
A technology of hydroxyethoxy and manufacturing methods, applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of high bulk density, low bulk density, etc., to improve the purity, reduce the manufacturing cost, Handling easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
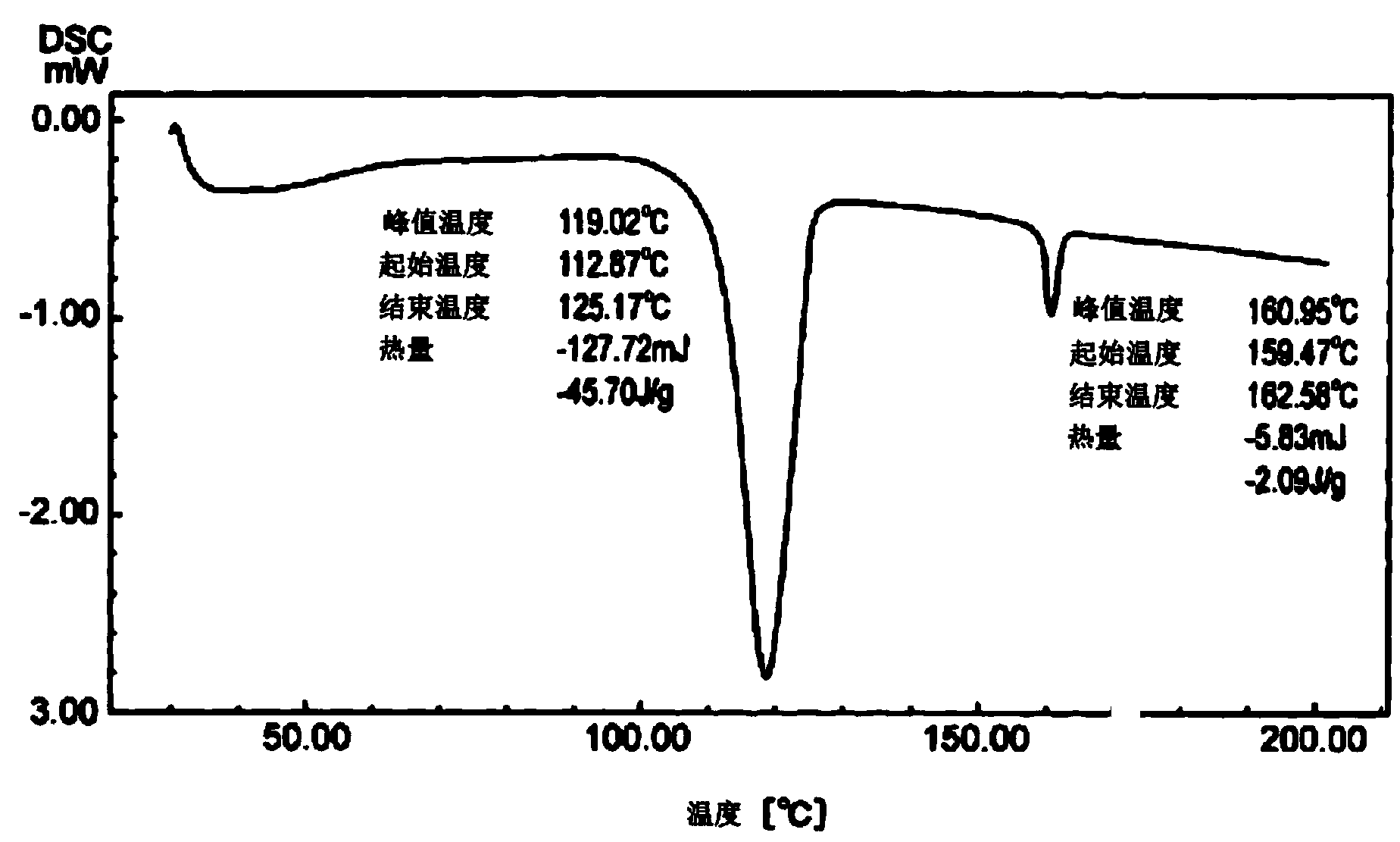
[0077] A four-necked flask equipped with a thermometer, a stirrer, and a cooling tube was charged with 544.7 g (3.94 mol) of 2-phenoxyethanol. After replacing the reaction vessel with nitrogen, 840.7 g (8.75 mol) of methanesulfonic acid was added at 40°C. After that, 3.8 g of β-mercaptopropionic acid was added at 50°C, and 347.9 g (1.93 mol) 9- was dissolved in 521.9 g (3.78 mol) of 2-phenoxyethanol at 50° C. for 1 hour. A solution of fluorenone for the reaction. After the dripping was completed, stirring was performed at 50°C for 17 hours. The reaction solution was analyzed by high performance liquid chromatography using a standard curve. As a result, the yield of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene present in the reaction solution was 85.4%.
[0078] After the completion of the reaction, 1287.2 g of toluene and 890.9 g of water were added at 50° C. while stirring, and then left to stand to extract 1664 g of the water layer. The concentration of methanesulfonic acid in ...
Embodiment 2
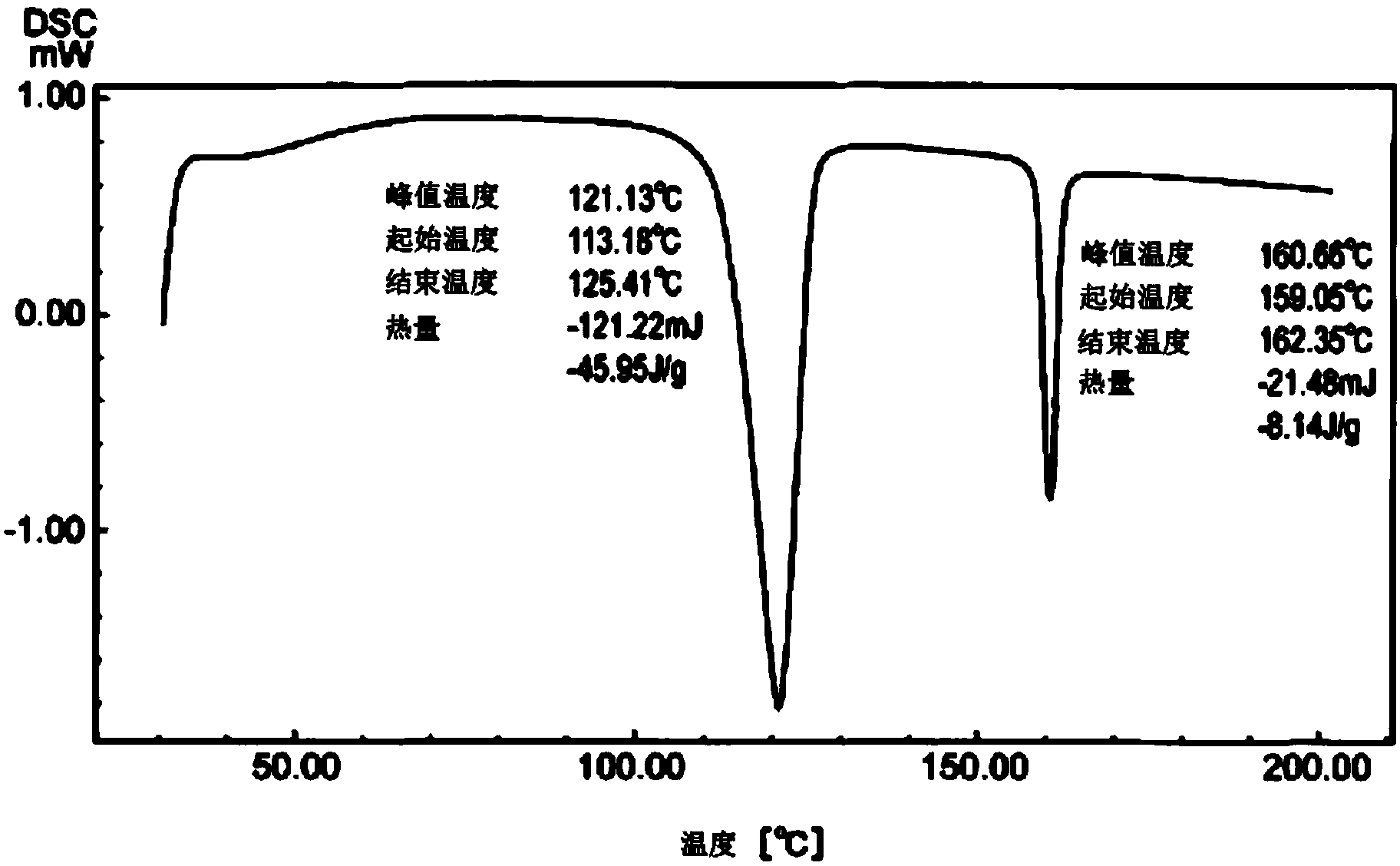
[0083] A four-necked flask equipped with a thermometer, a stirrer, and a cooling tube was charged with 60.0 g of crude crystals obtained in the same manner as in Example 1, and after nitrogen substitution, 60.0 g of 1-butanol and 60.0 g of toluene were added, and the temperature was raised to Dissolve at 70°C. Thereafter, a seed crystal of 9,9-bis[4-(2-hydroxyethoxy)phenyl]fluorene as a high melting point crystal was added at 60°C, and the crystal was precipitated while being held at 35°C for 30 minutes. After that, the temperature was raised to 50°C and slowly cooled to 25°C, and the precipitated crystals were filtered out. The obtained crystals were heated to 80°C under reduced pressure and dried to obtain 44.3 g of 9,9-bis[4-(2-hydroxyethoxy)phenyl]fluorene. The yield relative to the crude crystals was 74%, and the purity analyzed by high performance liquid chromatography was 99.2%. The DSC data of the obtained crystals are as follows figure 2 Shown.
[0084] Maximum endot...
Embodiment 3
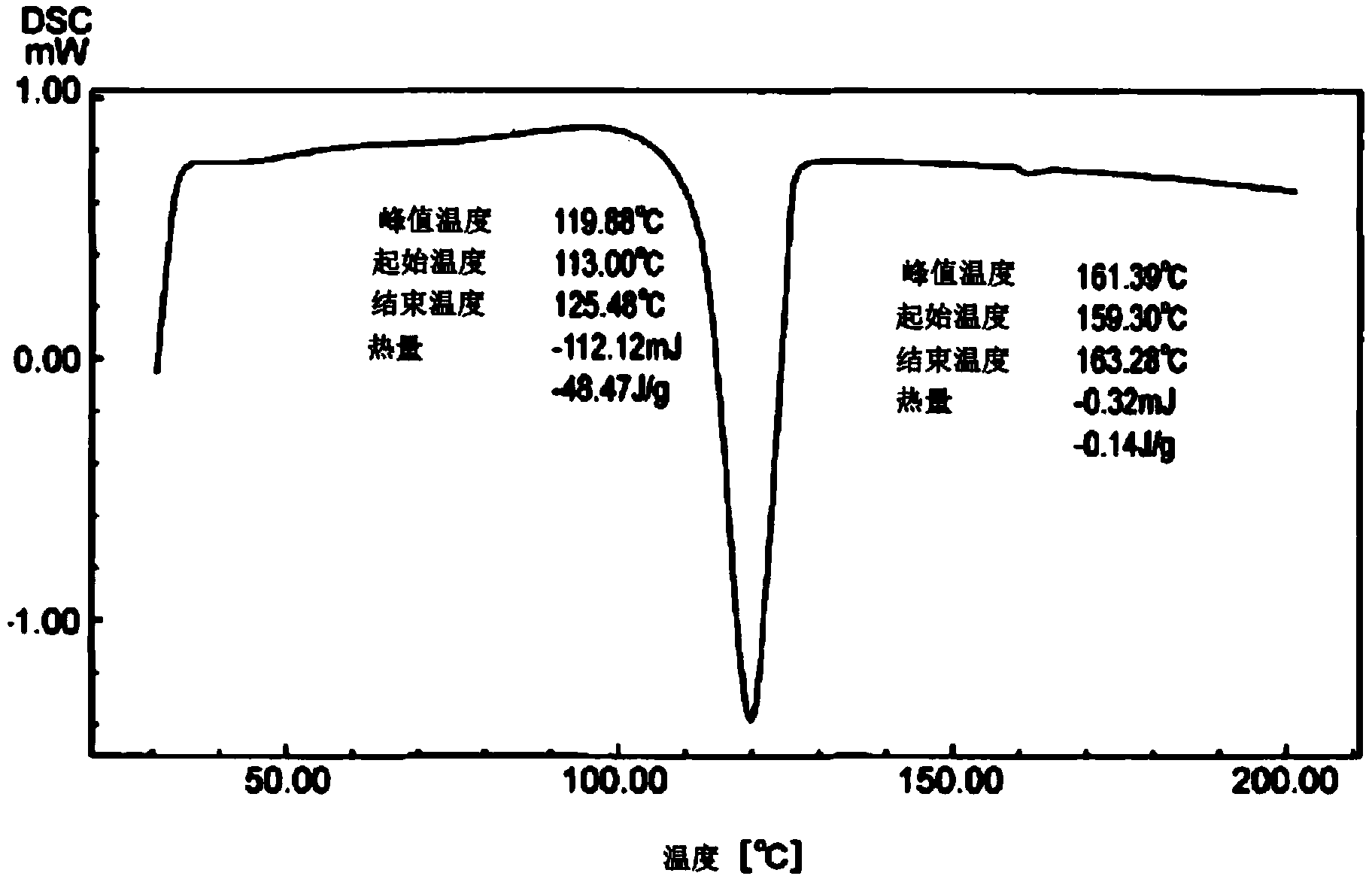
[0087] A four-necked flask equipped with a thermometer, a stirrer, and a cooling tube was charged with 60.0 g of the crude crystals obtained in the same manner as in Example 1. After nitrogen replacement, 40.0 g of 1-butanol and 80.0 g of toluene were added, and the temperature was raised to Dissolve at 70°C. Thereafter, a seed crystal of 9,9-bis[4-(2-hydroxyethoxy)phenyl]fluorene as a high melting point crystal was added at 60°C, and the crystal was precipitated while being held at 33°C for 30 minutes. After that, the temperature was raised to 50°C and slowly cooled to 25°C, and the precipitated crystals were filtered out. The obtained crystals were heated to 70°C under reduced pressure and dried to obtain 44.3 g of 9,9-bis[4-(2-hydroxyethoxy)phenyl]fluorene. The yield relative to the crude crystals was 75%, and the purity analyzed by high performance liquid chromatography was 99.0%. The DSC data of the obtained crystals are as follows image 3 Shown.
[0088] Maximum endoth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com