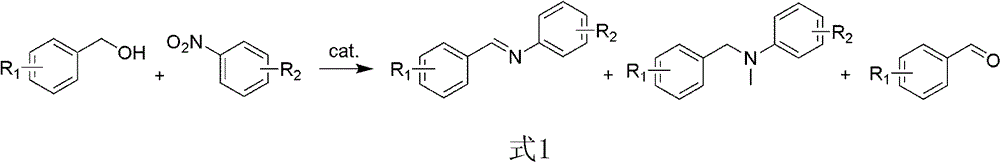
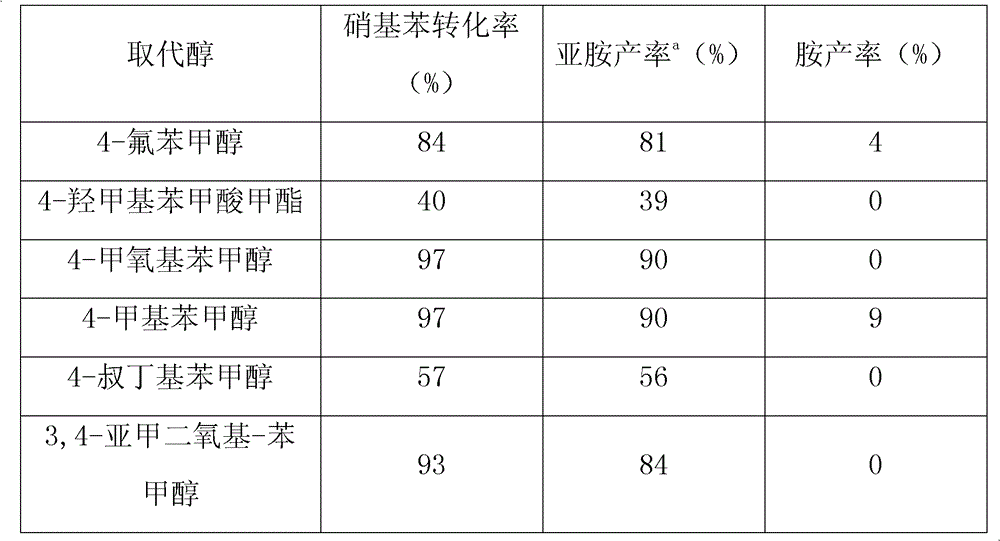
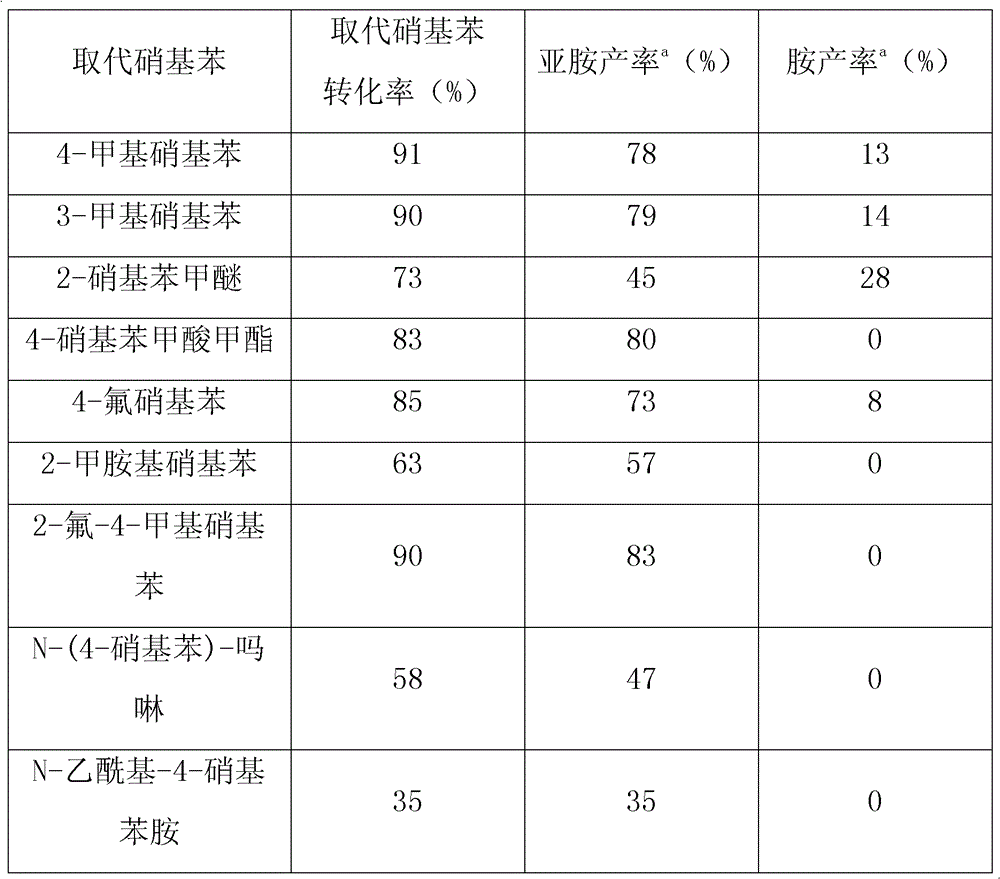
Method for synthesizing amine and imine
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of organic compounds, condensation/addition reaction to prepare amino compounds, etc., can solve problems such as reaching 22.3%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 0.0354gPdCl 2 , add 30 mL of deionized water to dissolve, add 2 mol / L hydrochloric acid to adjust the pH of the solution to 3, add 1 g of hydrotalcite HT to the above solution, stir at room temperature for 4 hours, and let stand for 10 hours. The mixture was evaporated to dryness at 45°C. The obtained powder was ground and dried at 120° C. for 10 hours to obtain a 2 wt % Pd / HT catalyst (Pd loading 0.2 mmol / g).
[0027] Catalyst performance evaluation was carried out in an atmospheric continuous reactor. Weigh 0.1g of 2wt% Pd / HT catalyst and add it into a 30mL reaction tube, add the reaction substrate benzyl alcohol 3mmol, nitrobenzene 1mmol, solvent toluene 2mL and internal standard n-dodecane 10μL under anaerobic conditions. The reaction temperature is controlled at 130 degrees Celsius by an oil bath. The above-mentioned reaction tube was placed in an oil bath with a stable temperature, and the reaction system was magnetically stirred at the same time, and the...
Embodiment 2
[0033] Weigh 0.0354gPdCl 2 , add 30 mL of deionized water to dissolve, add 2 mol / L hydrochloric acid to adjust the pH of the solution to 3, add 1 g of hydrotalcite HT to the above solution, stir at room temperature for 4 hours, and let stand for 10 hours. The mixture was evaporated to dryness at 45°C. The obtained powder was ground and dried at 120° C. for 10 hours to obtain a 2 wt % Pd / HT catalyst (Pd loading 0.2 mmol / g).
[0034] Catalyst performance evaluation was carried out in an atmospheric continuous reactor. Weigh 0.1g of 2wt% Pd / HT catalyst into a 30mL reaction tube, add 4mmol of benzyl alcohol, 1mmol of nitrobenzene, 2mL of solvent toluene and 10μL of internal standard n-dodecane under anaerobic conditions. The reaction temperature is controlled at 120 degrees centigrade by an oil bath. The above-mentioned reaction tube was placed in an oil bath with a stable temperature, and the reaction system was magnetically stirred at the same time, and the reaction timing st...
Embodiment 3
[0039] Weigh 0.0354gPdCl 2, add 30mL deionized water to dissolve, add 2mol / L hydrochloric acid to adjust the pH of the solution to 3, add 1g hydrotalcite HT to the above solution, stir at room temperature for 4 hours, and let stand for 10 hours. The mixture was evaporated to dryness at 45°C. The obtained powder was ground and dried at 120° C. for 10 hours to obtain a 2 wt % Pd / HT catalyst (Pd loading 0.2 mmol / g).
[0040] Catalyst performance evaluation was carried out in an atmospheric continuous reactor. Weigh 0.1g of 2wt% Pd / HT catalyst and add it into a 30mL reaction tube, add 15mmol of benzyl alcohol, 1mmol of nitrobenzene, solvent toluene and 10μL of internal standard n-dodecane under anaerobic conditions. The reaction temperature is controlled at 130 degrees Celsius by an oil bath. The above-mentioned reaction tube was placed in an oil bath with a stable temperature, and the reaction system was magnetically stirred at the same time, and the reaction timing started. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com