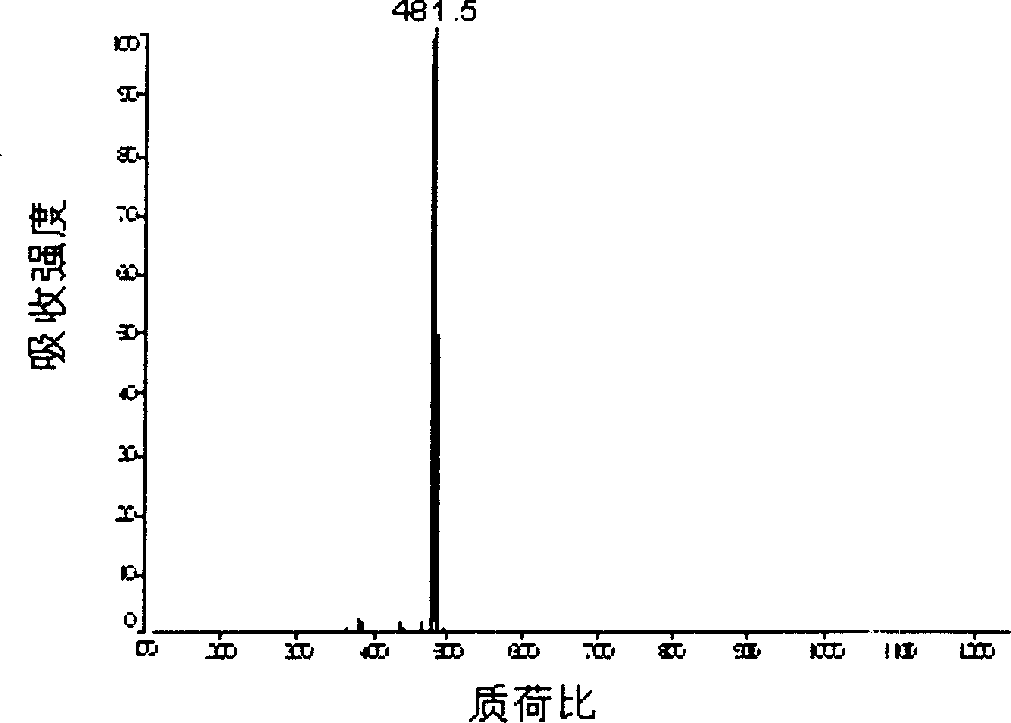
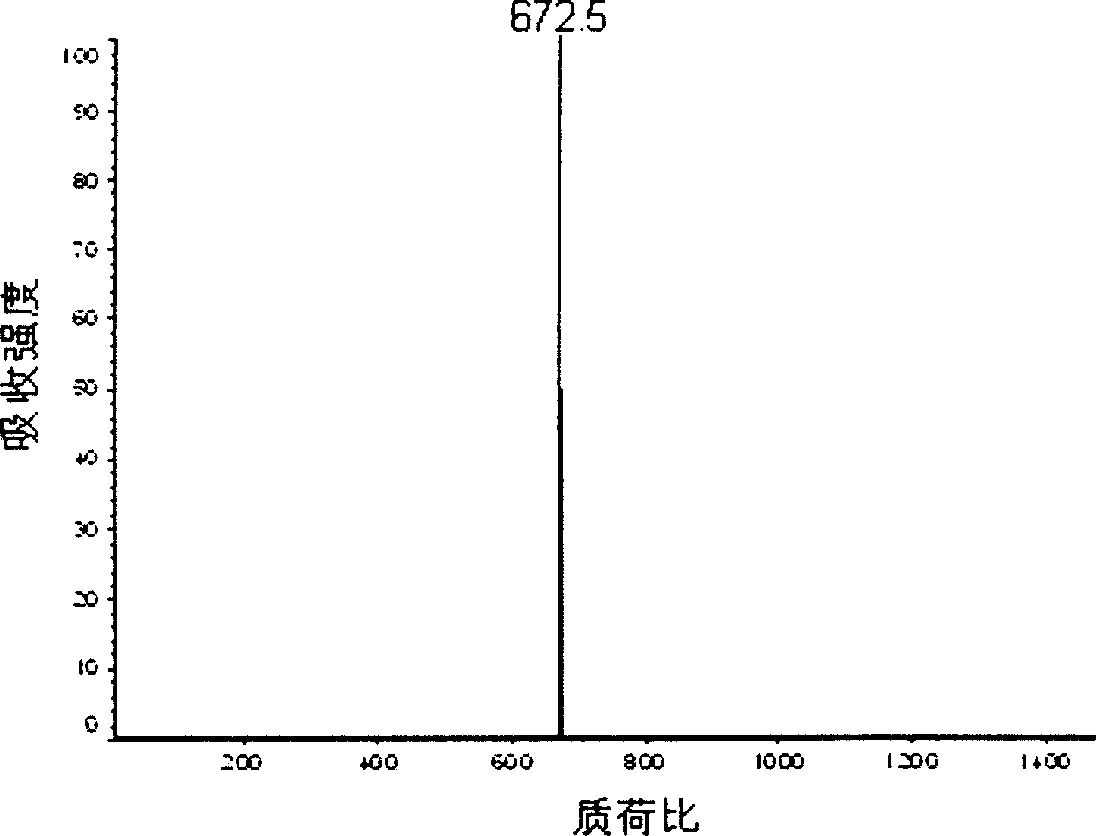
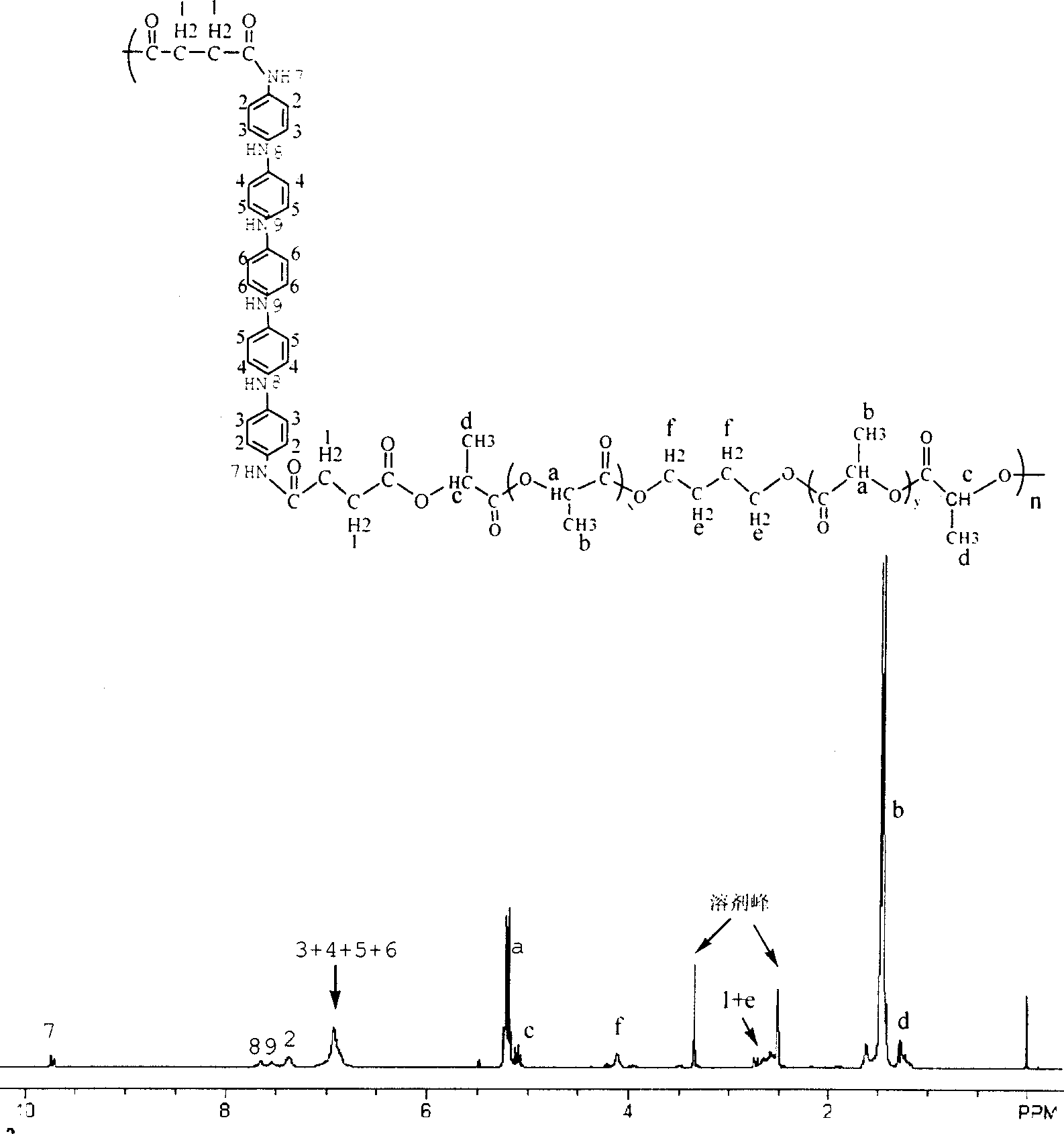
Aniline oligomer, its aliphatic polyester copolymer and their prepn
A technology of aliphatic polyesters and oligomers, applied in condensation/addition reactions to prepare amino compounds, organic chemistry, etc., can solve non-degradable problems, achieve convenient processing and utilization, good biocompatibility, and good bioavailability degradability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the synthesis of the aniline dimer of terminal amino protection
[0060] In a 500mL three-necked flask equipped with mechanical stirring, nitrogen inlet, and nitrogen outlet, add N-phenyl-1,4-p-phenylenediamine 9.21g (0.05mol), succinic anhydride 5.00g (0.05mol), Dichloromethane 300mL, while stirring. As the reaction proceeded, a gray precipitate was produced. After the reaction was completed, it was filtered, and the obtained precipitate was washed with ether until the filtrate was colorless. The sample was dried in a vacuum oven for 12 hours to obtain the amino-terminated aniline dimer with a yield of 84.5%.
Embodiment 2
[0061] Example 2: Synthesis of aniline tetramers with one carboxyl group and one amino group at the end
[0062] 2.85g (0.01mol) of aniline dimer and 3.5g (0.01mol) of amino-terminated dimer are dissolved in the mixed solution (comprising 100mlDMF, 15ml distilled water, 15ml concentrated hydrochloric acid), cooled to below zero, and then The hydrochloric acid solution of ammonium persulfate (2.28g dissolved in 50ml 1M hydrochloric acid) was slowly added to the above solution through the dropping funnel, while stirring rapidly, the ice salt kept the reaction below zero, after the dropwise addition was completed, the reaction was continued for 2h, and then the The solution was poured into 700ml of distilled water and filtered. Dissolve the obtained precipitate in 300ml of 1M ammonia water, add a hydration trap and stir for reduction overnight, then add an appropriate amount of 1M hydrochloric acid to adjust the pH to 2-3, filter, and vacuum dry the precipitate at 35°C for 48 hou...
Embodiment 3
[0063] Embodiment 3: the synthesis of dicarboxy-terminated aniline pentamer
[0064] Dissolve 2.85 g (0.01 mol) of amino-terminated aniline dimer and 0.54 g (0.005 mol) of p-phenylenediamine in 15 mL of DMF. Add 60 mL of pre-cooled mixed solution (including 30 mL of DMF, 25 mL of distilled water, and 5 mL of concentrated hydrochloric acid), then slowly add a hydrochloric acid solution of ammonium persulfate (2.28 g in 50 mL of 1 mol / L hydrochloric acid) into the above solution through a dropping funnel, Stir rapidly at the same time, after the dropwise addition is completed, continue to react for 1 h, then pour the solution into 300 mL of distilled water and filter. Dissolve the obtained precipitate in 300mL of 1mol / L ammonia water, add hydrazine hydrate and stir for reduction overnight, then add an appropriate amount of 1mol / L hydrochloric acid to adjust the pH to 2-3, filter, and vacuum-dry the precipitate at 45°C for 48h . The obtained 3.0g sample was dissolved in 15mL DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com