Solid acid catalyst and preparation method and applications thereof
A solid acid catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Low-level problems, high reactivity and selectivity, easy to realize the preparation method, and high tert-butylamine selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
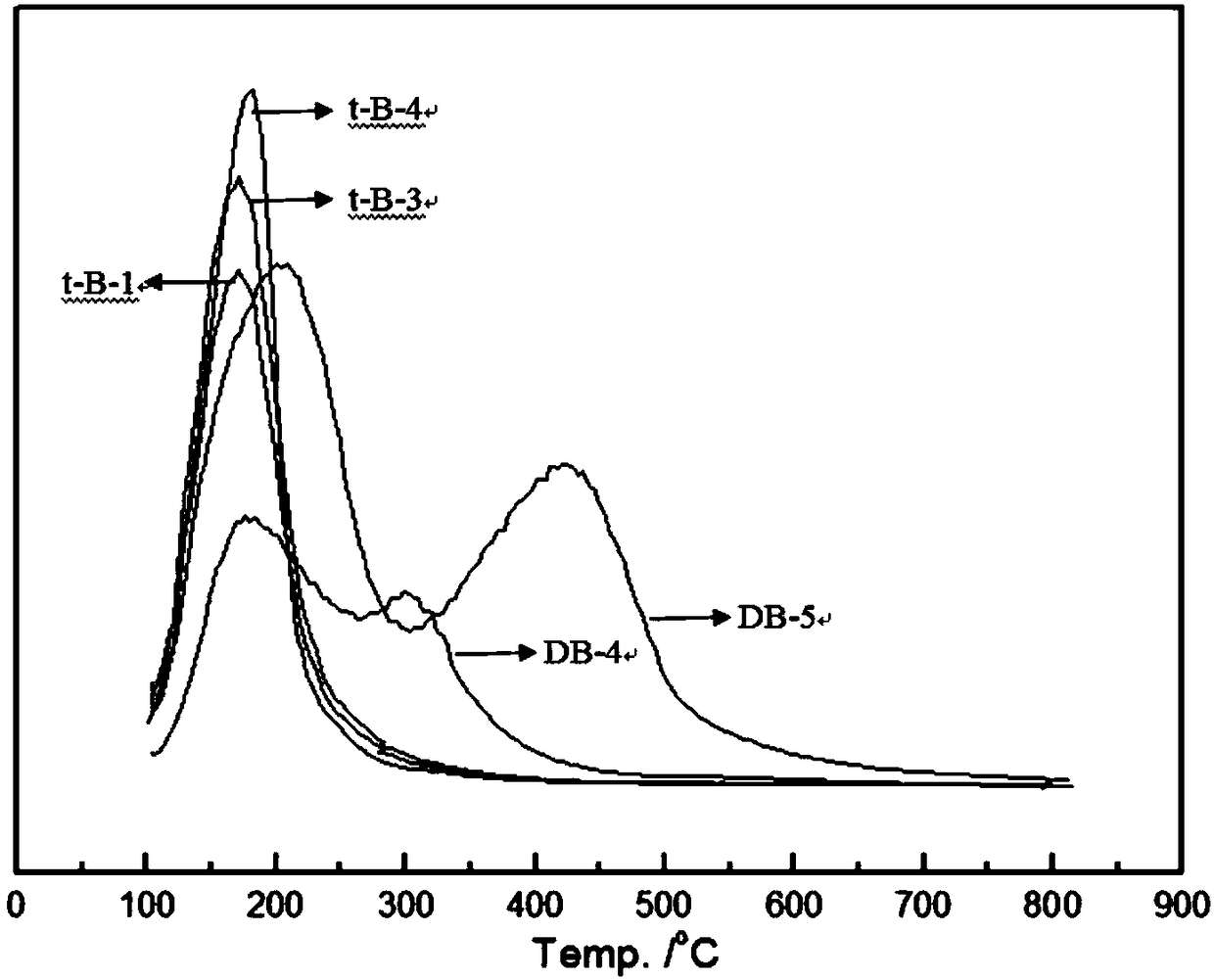
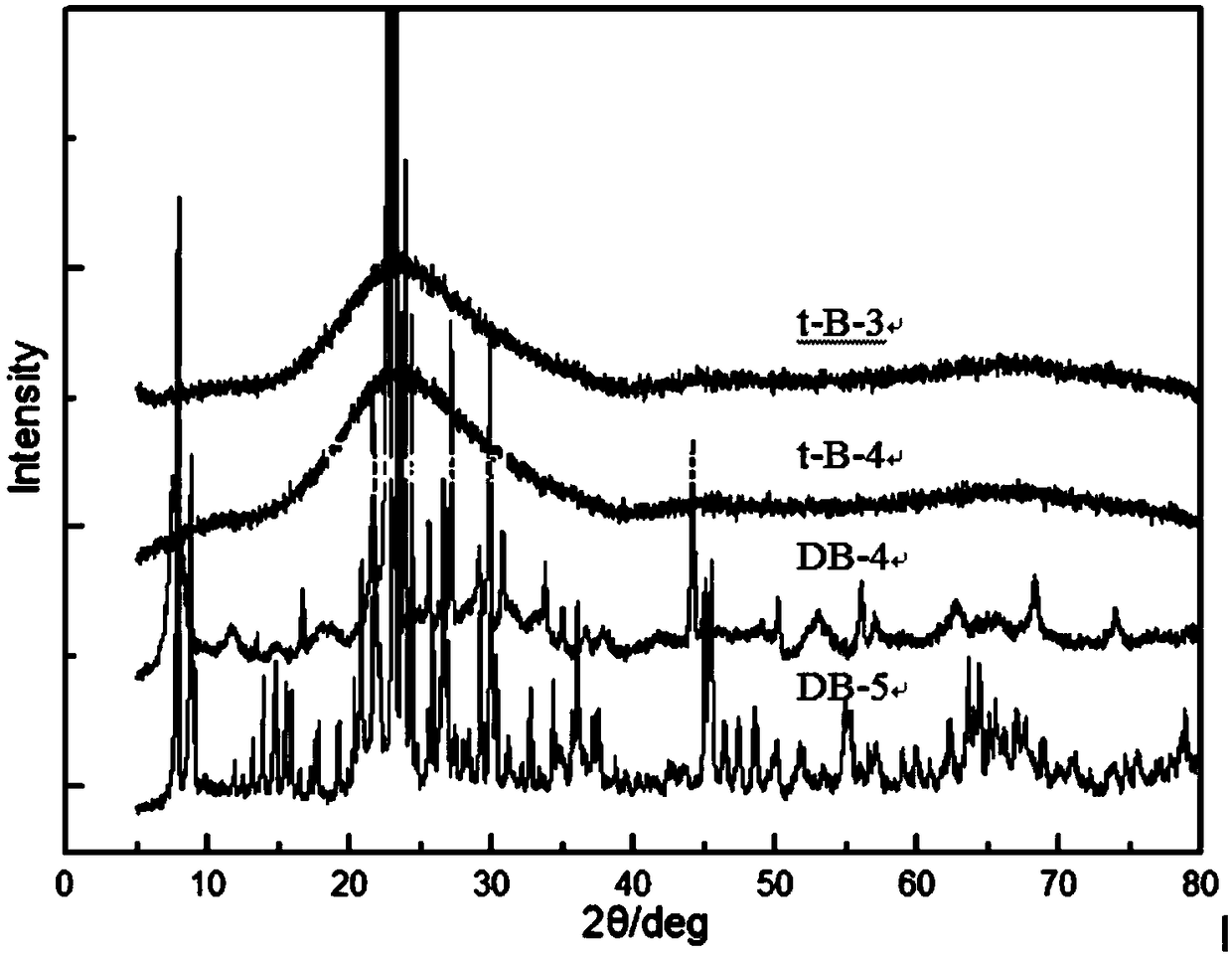
Image
Examples
Embodiment 1
[0068] (1) Preparation of borosilicate composite oxide
[0069] Add 300g tetraethyl orthosilicate and 200g ethanol into a 1L hydrothermal kettle, adjust the stirring speed to 300r / min at room temperature, add 6.5g CTAB (cetyltrimethylammonium bromide) and stir for 1h; add 2.97g boric acid Dissolve in 15g of deionized water, increase the stirring speed of the hydrothermal kettle to 3000r / min, slowly add the boric acid solution into the hydrothermal kettle, and stir vigorously for 1.5h. Reduce the stirring speed to 100r / min, add HNO dropwise 3 Adjust the pH to 6-6.5, and age at 50°C for 12 hours.
[0070] Take 100 g of the prepared gel and transfer it to a high-pressure stainless steel hydrothermal kettle, add 50 ml of ammonia solution with a concentration of 36 wt%, and treat it at a constant temperature at 80° C. for 6 hours. Dry the gel treated with ammonia water at 120°C for 3 hours, and then bake it at 550°C for 3 hours to obtain an amorphous borosilicate composite oxide ...
Embodiment 2
[0077] 15.5g La(NO 3 ) 3 ·6H 2 O was dissolved in 200g of deionized water to form an impregnating solution and added to the flask of a rotary evaporator, and 100g of the catalyst precursor prepared in Example 1 was added, and rotated and impregnated at 60°C for 3h to carry out ion exchange reaction; Down drying 5h, obtains the catalyst precursor of La modification;
[0078] Then the La-modified catalyst precursor was moved into the tubular roaster, and N 2 and programmed temperature up to 400°C, N 2 The volume space velocity is 75h -1 , after reaching 400°C the pure N 2 Switch to HBr and N 2 The mixed gas, the HBr concentration is 10v%, the activation treatment is 3h, after the treatment is completed, switch back to pure N 2 Purging was performed for 6 hours, and the temperature was lowered to room temperature to obtain a 5wt% La-3wt% Cl modified catalyst, which was denoted as t-B-2 based on the weight of the carrier.
Embodiment 3
[0080] Add 300g tetraethyl orthosilicate and 200g ethanol into a 1L hydrothermal kettle, adjust the stirring speed to 300r / min at room temperature, add 6.5g CTAB and stir for 1h; add 8.6g NaBO 2 Dissolve in 50g of deionized water, increase the stirring speed of the hydrothermal kettle to 3000r / min, slowly add the sodium metaborate solution into the hydrothermal kettle, and stir vigorously for 1.5h. Raise the temperature of the hydrothermal kettle to 60°C, reduce the stirring speed to 100r / min, add HNO dropwise 3 Adjust the pH to 6-6.5, and age at 50°C for 12 hours.
[0081] Transfer 100 g of the prepared gel to a high-pressure stainless steel hydrothermal kettle, add 20 g of liquid ammonia, and treat at a constant temperature of 110 ° C for 6 h. Dry the gel treated with liquid ammonia at 120°C for 3 hours, and then bake it at 550°C for 3 hours to obtain an amorphous borosilicate composite oxide, the composition of which is Na 2 O·B 2 o 3 22SiO2 2 ·(H 2 O) x , the specif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com