Patents
Literature
1279results about "By adsorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
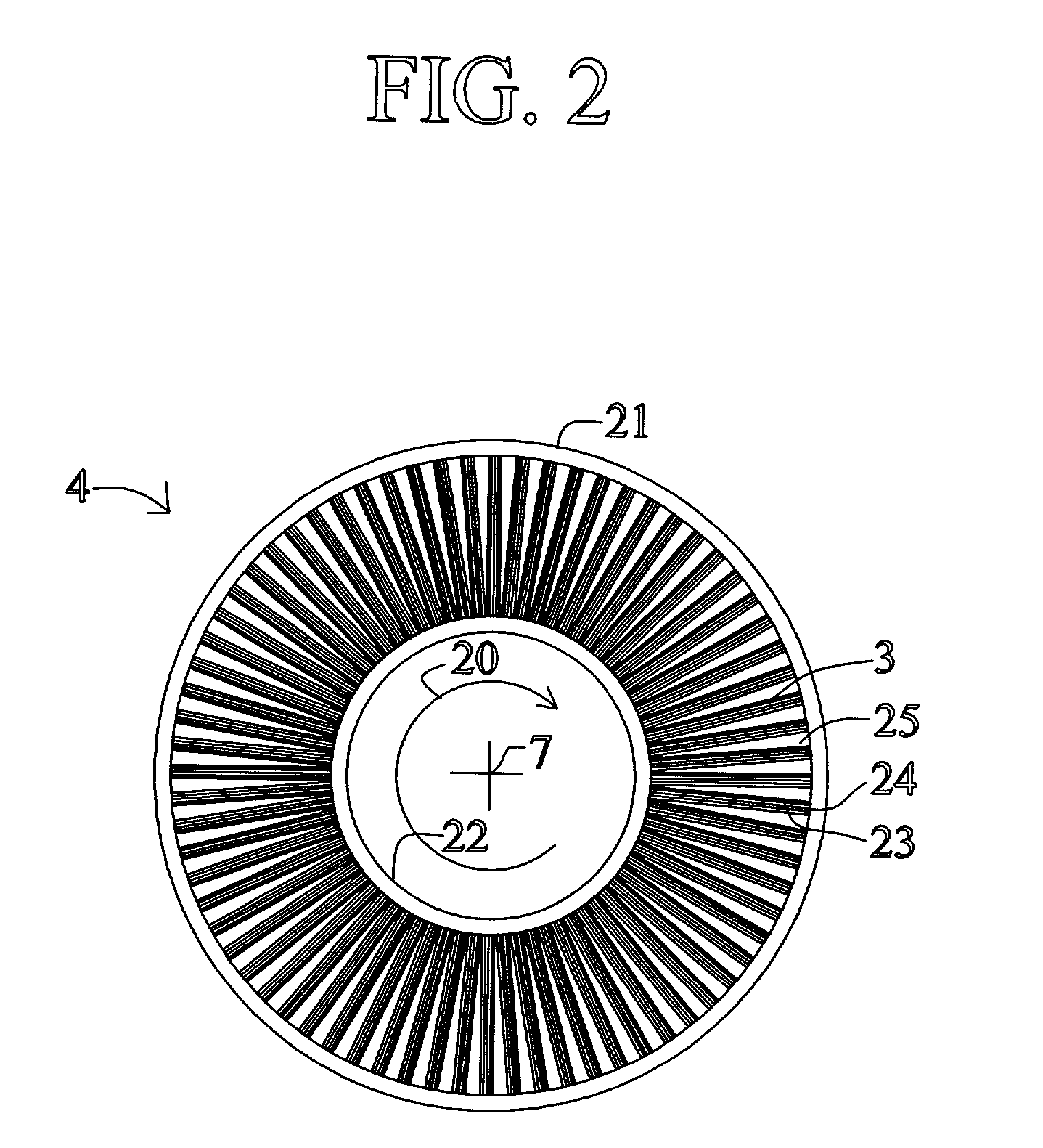
High frequency pressure swing adsorption
InactiveUS6176897B1High purityRecoverable expansion workNitrogen purification/separationGas treatmentSorbentEngineering
Pressure swing adsorption separation of a feed gas mixture, to obtain a purified product gas of the less strongly adsorbed fraction of the feed gas mixture, is performed in a plurality of preferably an even number of adsorbent beds, with each adsorbent bed communicating at its product end directly to a variable volume expansion chamber, and at its feed end by directional valves to a feed compressor and an exhaust vacuum pump. For high frequency operation of the pressure swing adsorption cycle, a high surface area layered support is used for the adsorbent. The compressor and vacuum pump pistons may be integrated with the cycle, reciprocating at twice the cycle frequency. Alternative configurations of the layered adsorbent beds are disclosed.
Owner:AIR PROD & CHEM INC
Conductive lithium storage electrode
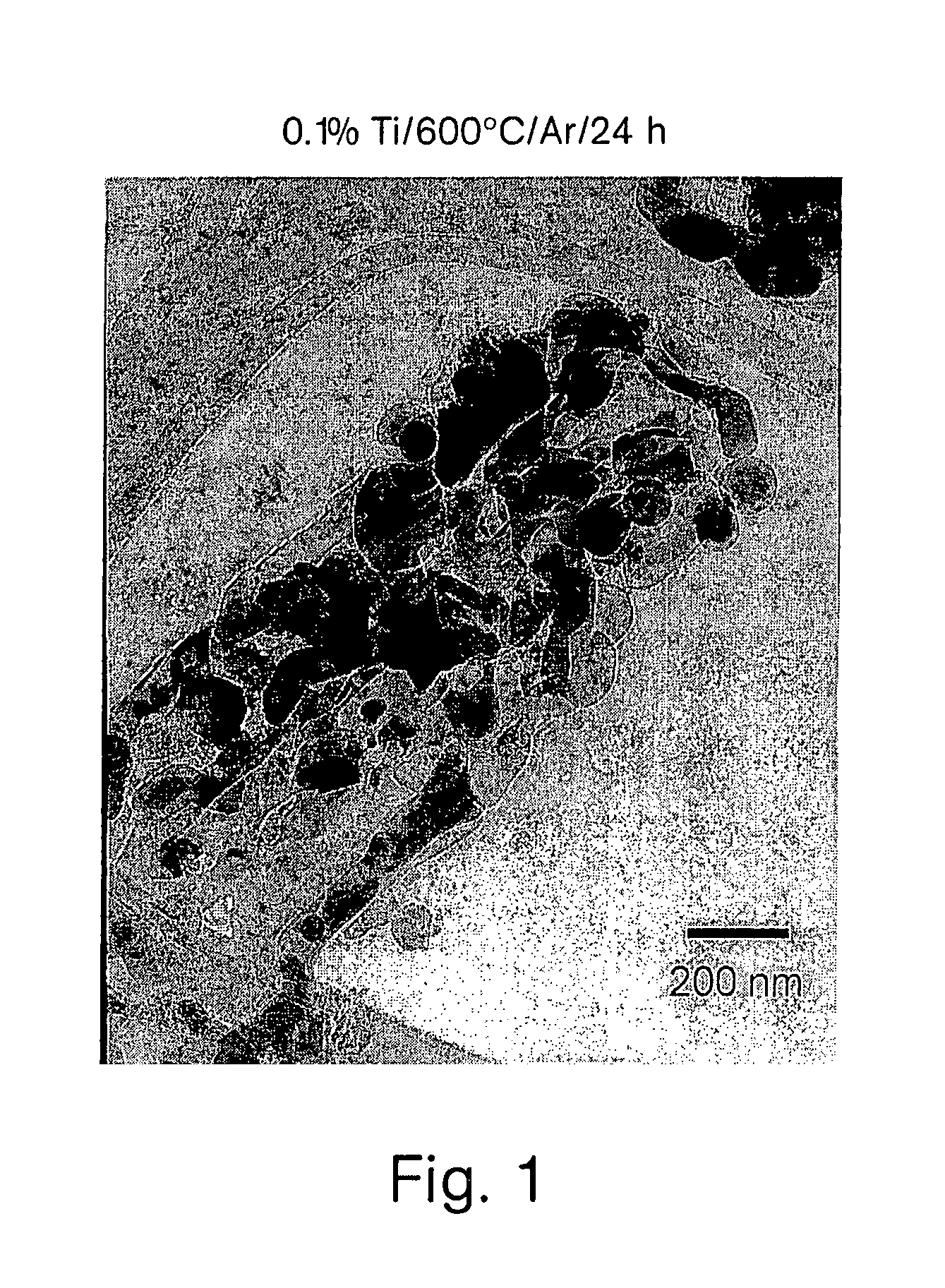
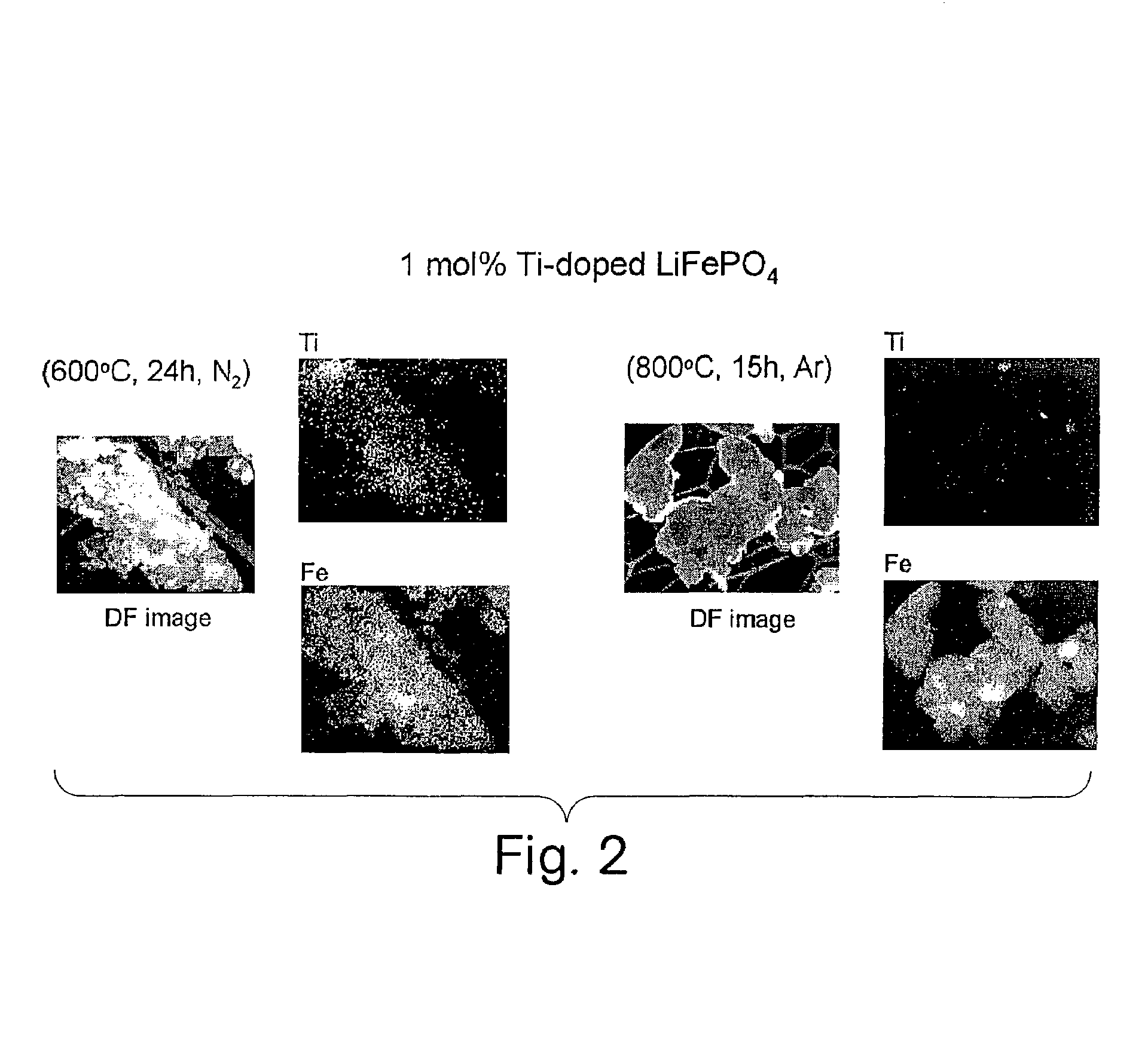
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
Sequestration of carbon dioxide
ActiveUS7132090B2Safe storageLarge specific surface areaCalcium/strontium/barium carbonatesProductsProduct gasMineral ions
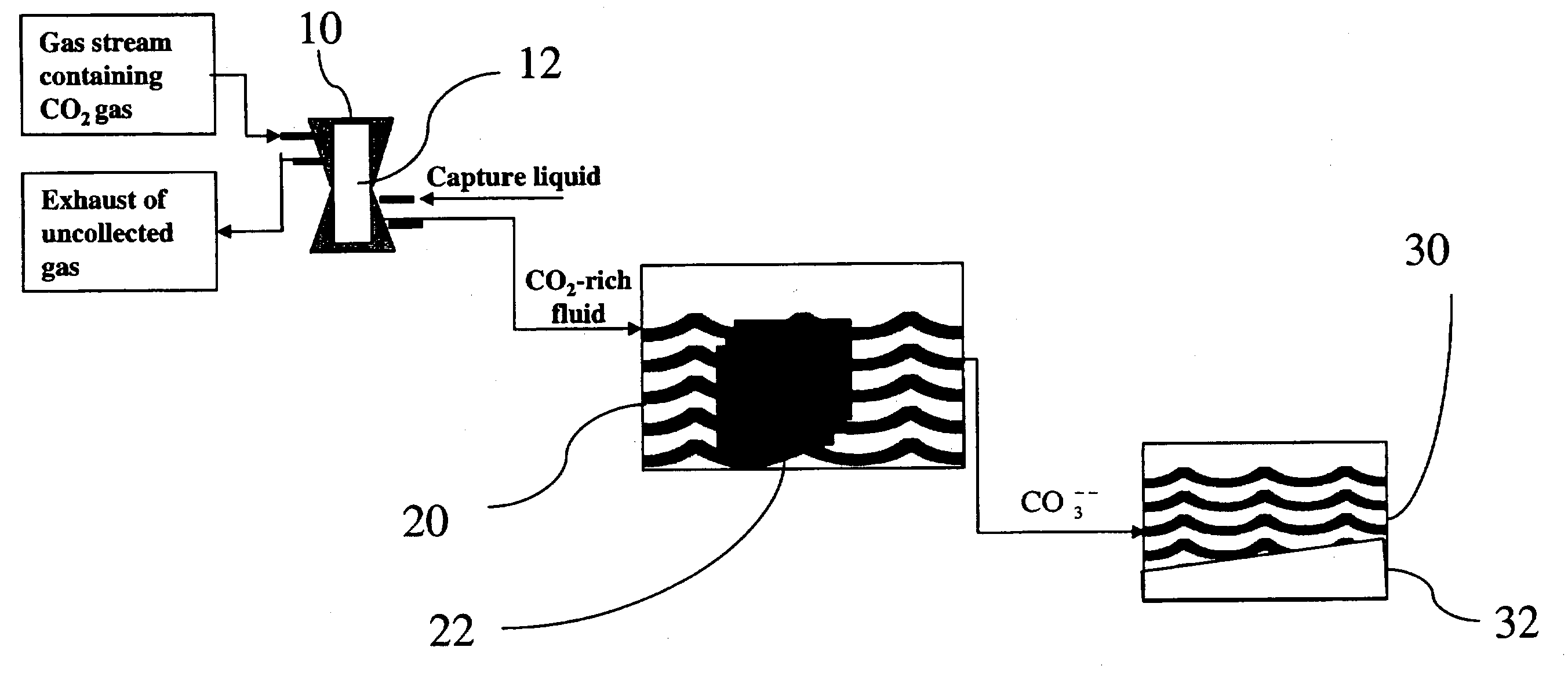
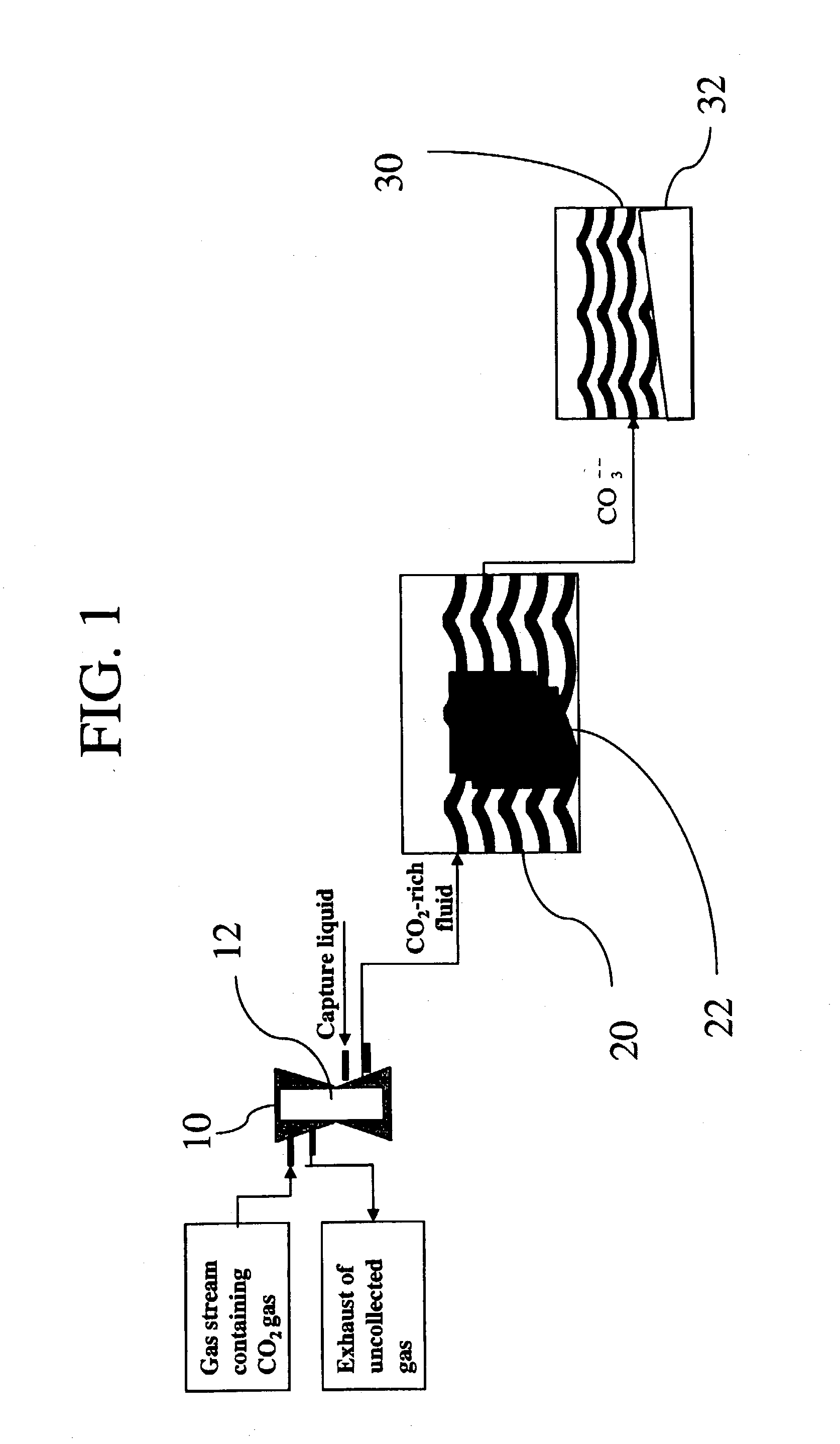
A process for selectively removing carbon dioxide from a gaseous stream by converting the carbon dioxide to a solid, stable form is provided. In a sequestration process, carbon dioxide enriched air is passed through a gas diffusion membrane to transfer the carbon dioxide to a fluid medium. The carbon dioxide rich fluid is then passed through a matrix containing a catalyst specific for carbon dioxide, which accelerates the conversion of the carbon dioxide to carbonic acid. In the final step, a mineral ion is added to the reaction so that a precipitate of carbonate salt is formed. This solid mineral precipitate can be safely stored for extended periods of time, such as by burying the precipitate in the ground or depositing the precipitate into storage sites either on land or into a body of water. An apparatus for removing carbon dioxide from a gaseous stream is also provided.
Owner:GM GLOBAL TECH OPERATIONS LLC
Low mesopore adsorbent contactors for use in swing adsorption processes
The present invention relates to engineered structured adsorbent contactors for use in pressure swing adsorption and thermal swing adsorption processes. Preferably, the contactors contain engineered and substantially parallel flow channels wherein 20 volume percent or less of the open pore volume of the contactor, excluding the flow channels, is in the mesopore and macropore range.
Owner:EXXON RES & ENG CO
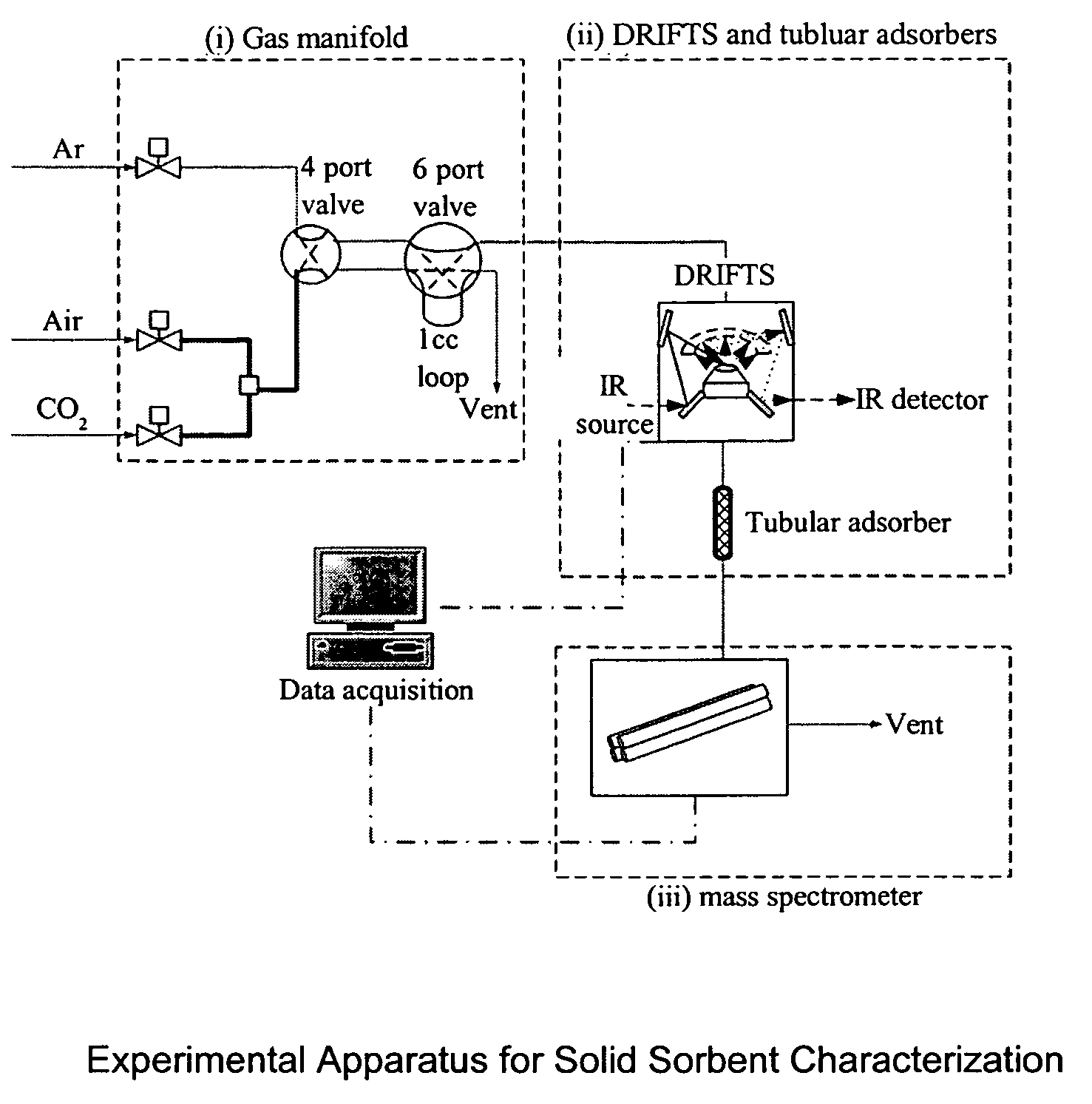
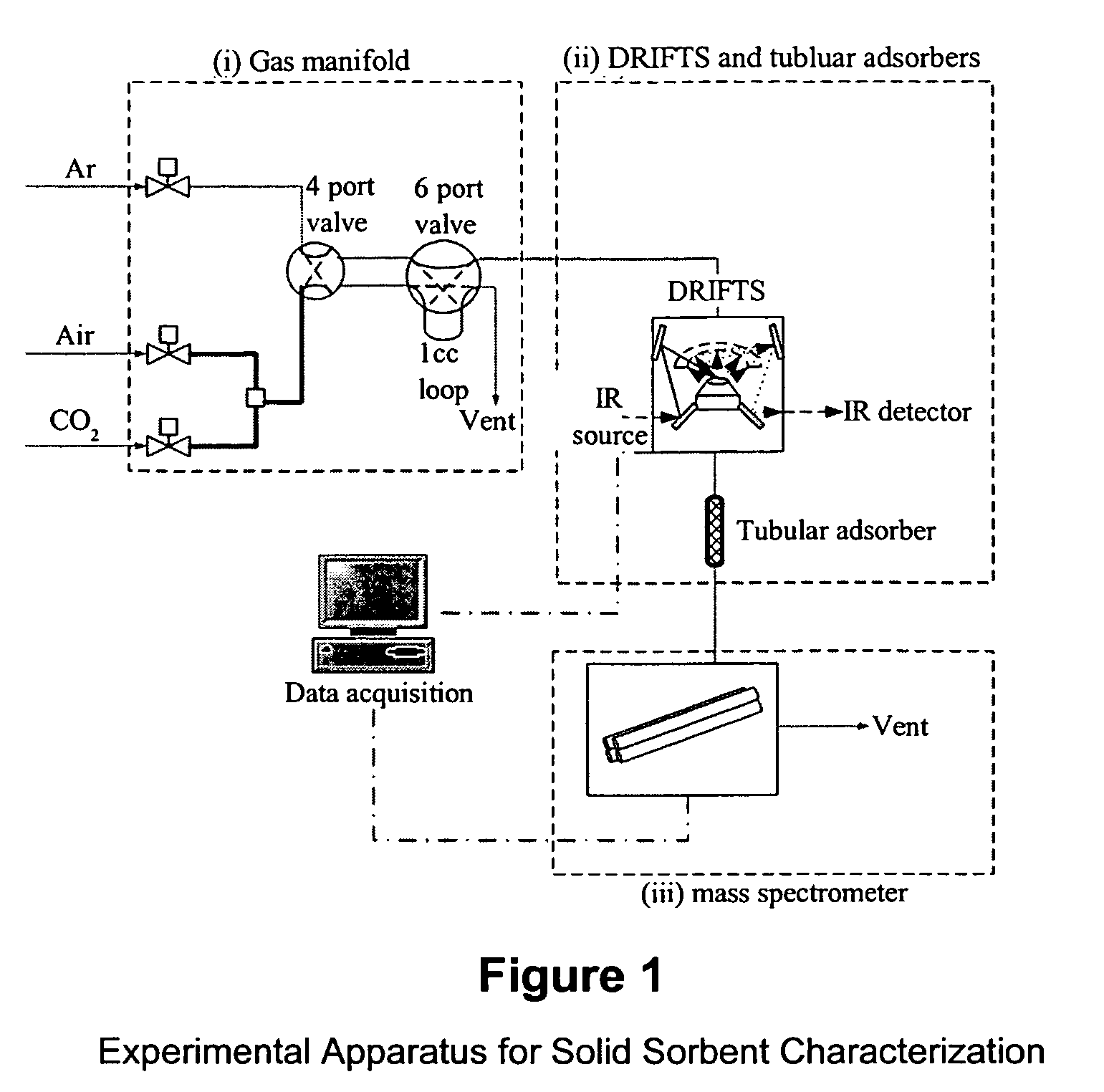
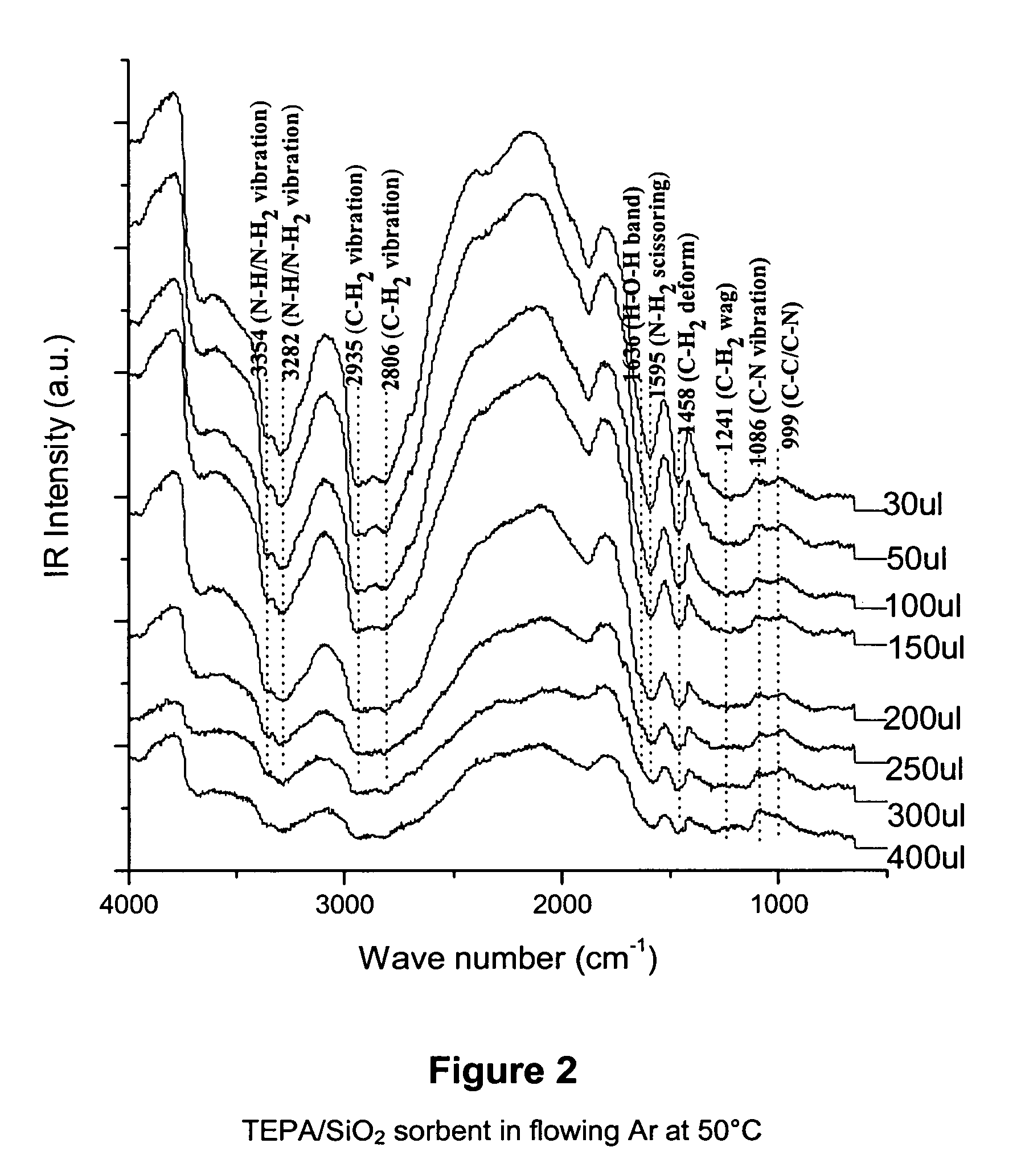
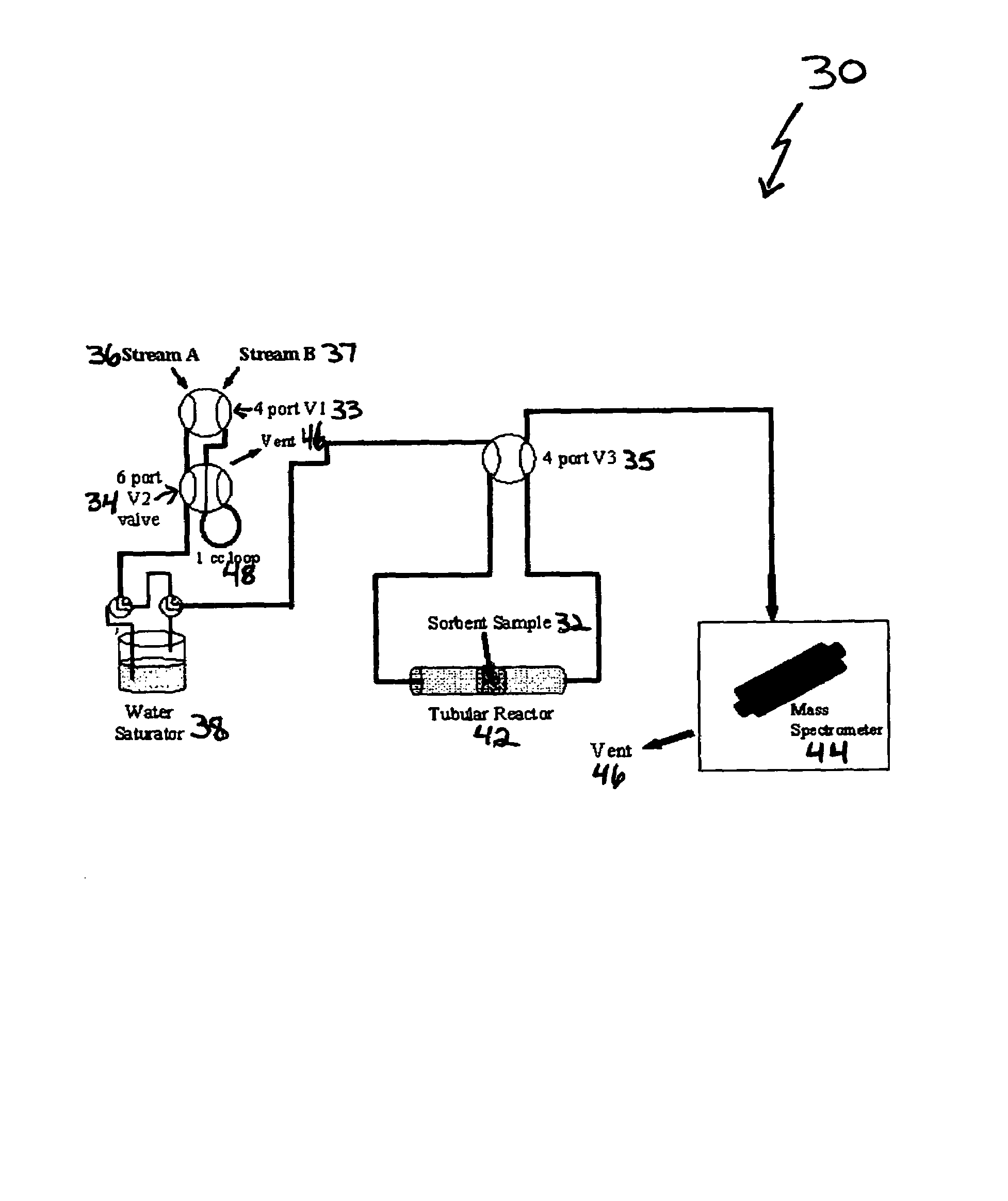
Solid sorbents for removal of carbon dioxide from gas streams at low temperatures
InactiveUS6908497B1Promote regenerationRegeneration process is inexpensiveGas treatmentOther chemical processesGas solidSorbent
New low-cost CO2 sorbents are provided that can be used in large-scale gas-solid processes. A new method is provided for making these sorbents that involves treating substrates with an amine and / or an ether so that the amine and / or ether comprise at least 50 wt. percent of the sorbent. The sorbent acts by capturing compounds contained in gaseous fluids via chemisorption and / or physisorption between the unit layers of the substrate's lattice where the polar amine liquids and solids and / or polar ether liquids and solids are located. The method eliminates the need for high surface area supports and polymeric materials for the preparation of CO2 capture systems, and provides sorbents with absorption capabilities that are independent of the sorbents' surface areas. The sorbents can be regenerated by heating at temperatures in excess of 35° C.
Owner:ENERGY U S DEPARMENT OF
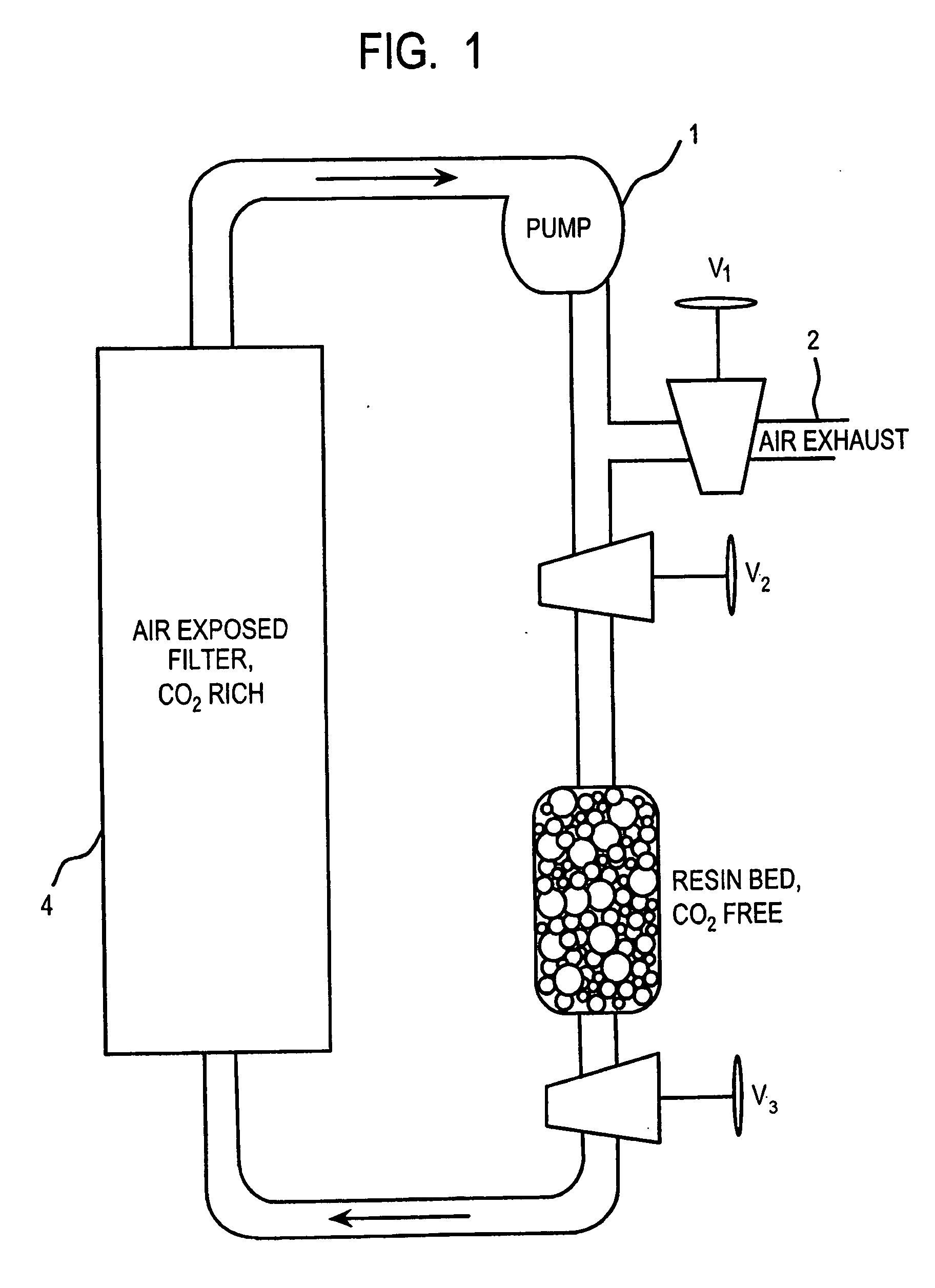
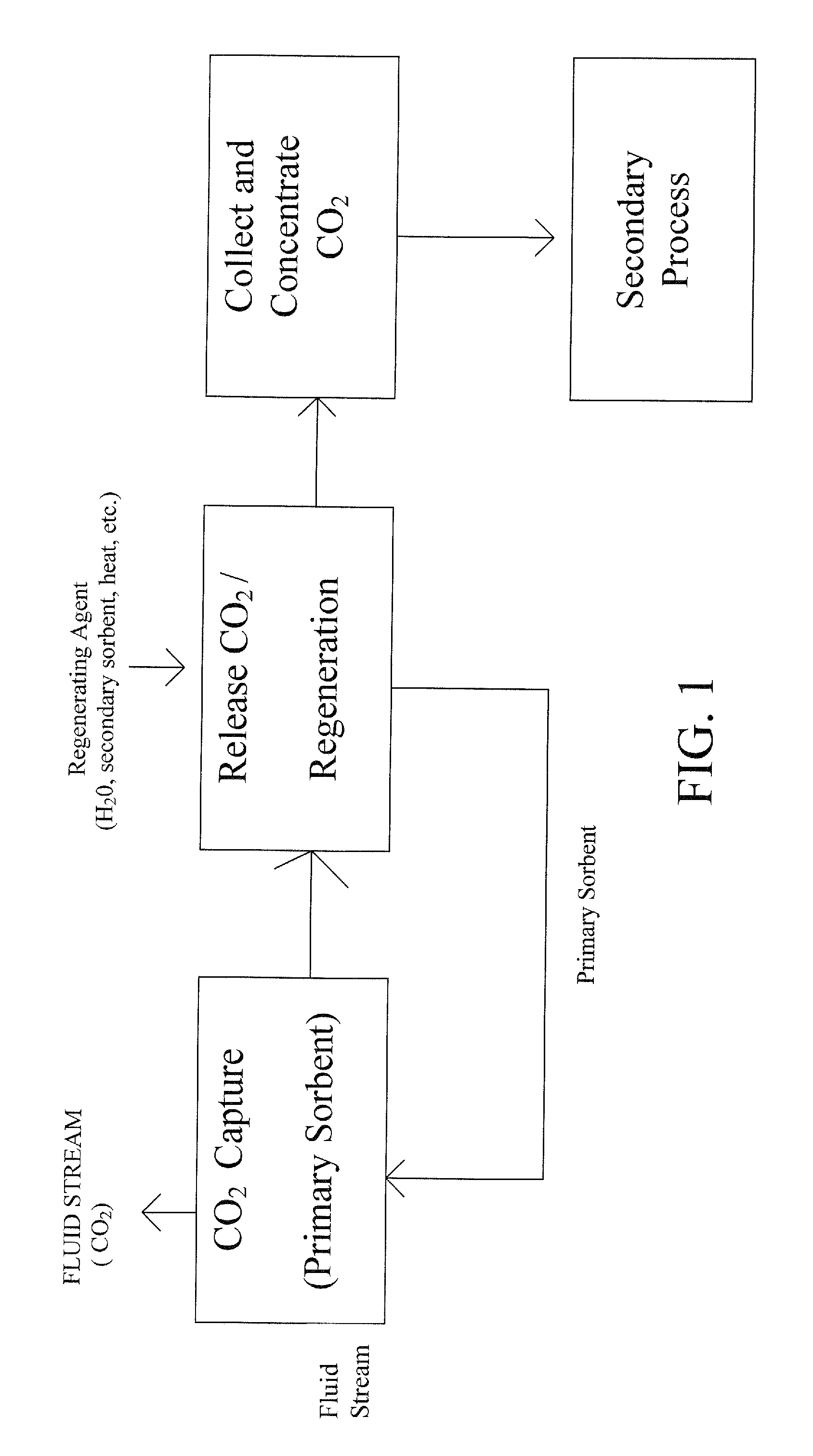
Method and apparatus for extracting carbon dioxide from air
A method and apparatus for extracting CO2 from air comprising an anion exchange material formed in a matrix exposed to a flow of the air, and for delivering that extracted CO2 to controlled environments. The present invention contemplates the extraction of CO2 from air using conventional extraction methods or by using one of the extraction methods disclosed; e.g., humidity swing or electro dialysis. The present invention also provides delivery of the CO2 to greenhouses where increased levels of CO2 will improve conditions for growth. Alternatively, the CO2 is fed to an algae culture.
Owner:CARBON SINK
Method for preparing loading functional oxide porous carbon
InactiveCN101780952AThe shape is fine and differentFine and varied structureOther chemical processesBy adsorptionPorous carbonMaterials science
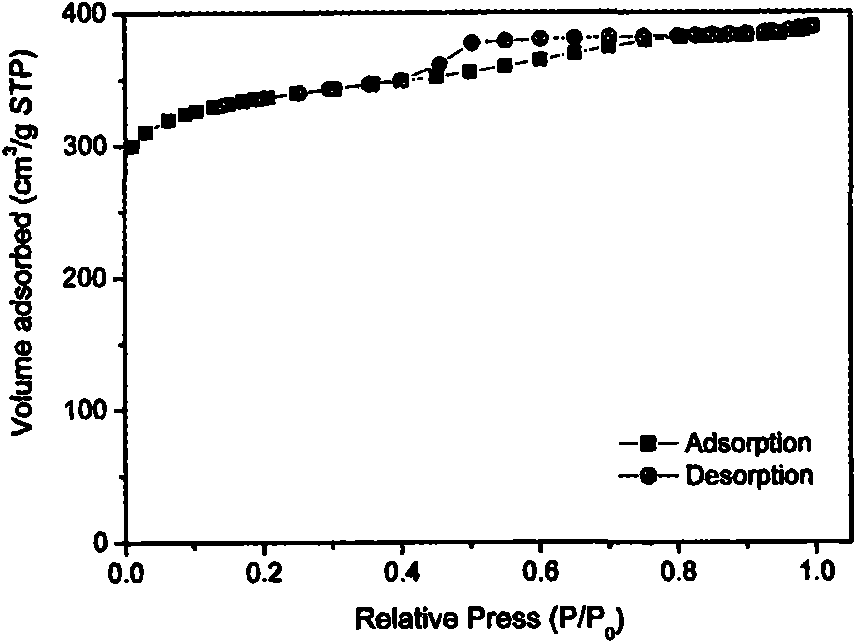
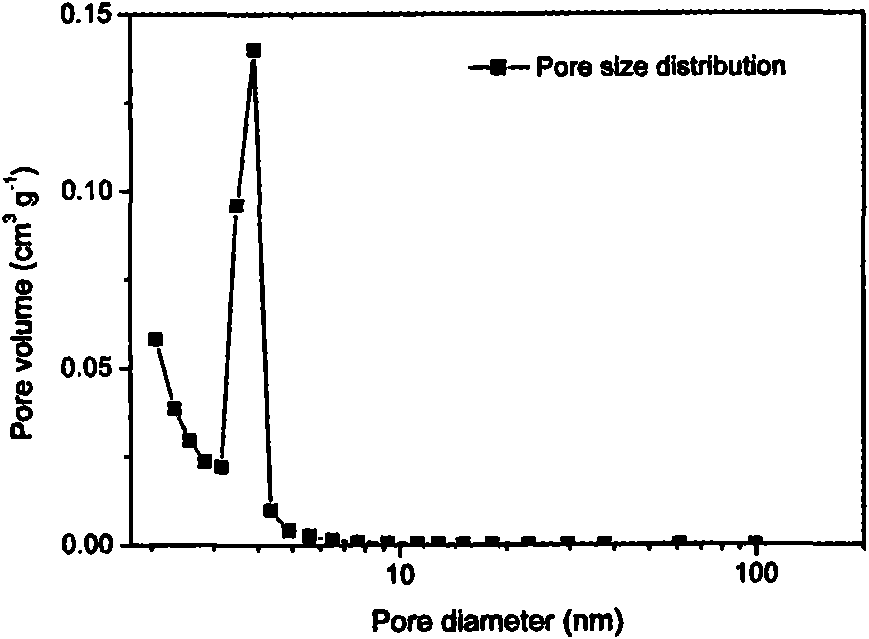
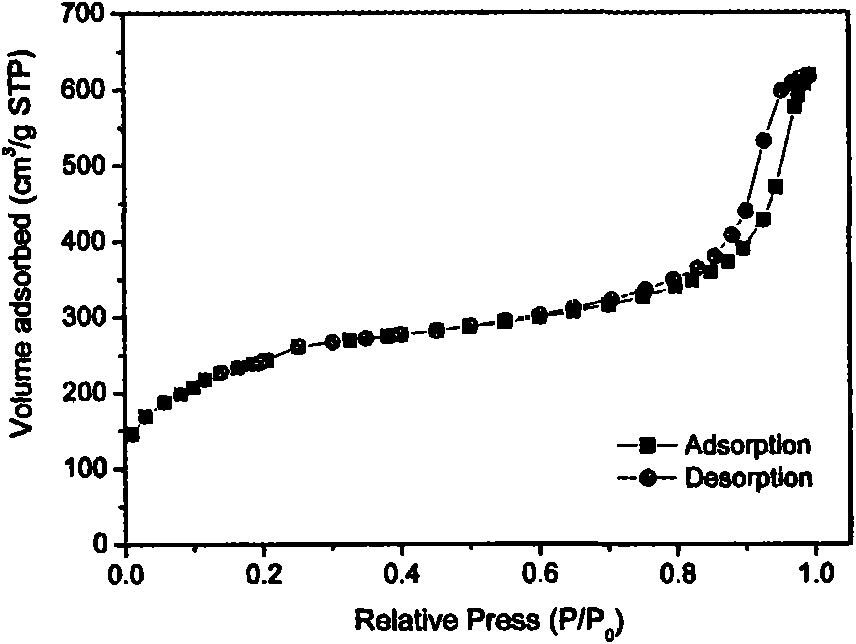
The invention relates to a method for preparing a loading functional oxide porous carbon, which belongs to the technical field of carbon material. The method comprises the following steps of: selecting biomass material, and carbonizing at the temperature of 400-1000 DEG C in vacuum or on inert condition; preparing porous carbon by a physical activating or chemical activating process according to the requirement on the pore structure by the carbonized porous carbon; immersing the porous carbon into a precursor solution of an element M, cleaning many times with distilled water, and drying; roasting on the porous carbon at the temperature of 300-1000 DEG C in vacuum or on inert atmosphere, and then the active carbon of the loading functional oxide MxOy is prepared. The active carbon of the loading functional oxide MxOy prepared by the invention has the special pore structure of the biomass, also has the functions performed by the oxides, and has important application value in water treatment, hydrogen storage, photocatalysis, fuel cells and relevant fields.
Owner:SHANGHAI JIAO TONG UNIV
Removal of CO2, N2, or H2S from gas mixtures by swing adsorption with low mesoporosity adsorbent contactors
The present invention relates to the separation of one or more of CO2, N2, and H2S gas components from a gas mixture containing at least a second gas using a swing adsorption process unit. The adsorbent contactors of the swing adsorption process unit are engineered structured adsorbent contactors having a plurality of flow channels wherein 20 volume percent or less of the open pore volume of the contactors is in the mesopore and macropore range.
Owner:EXXON RES & ENG CO
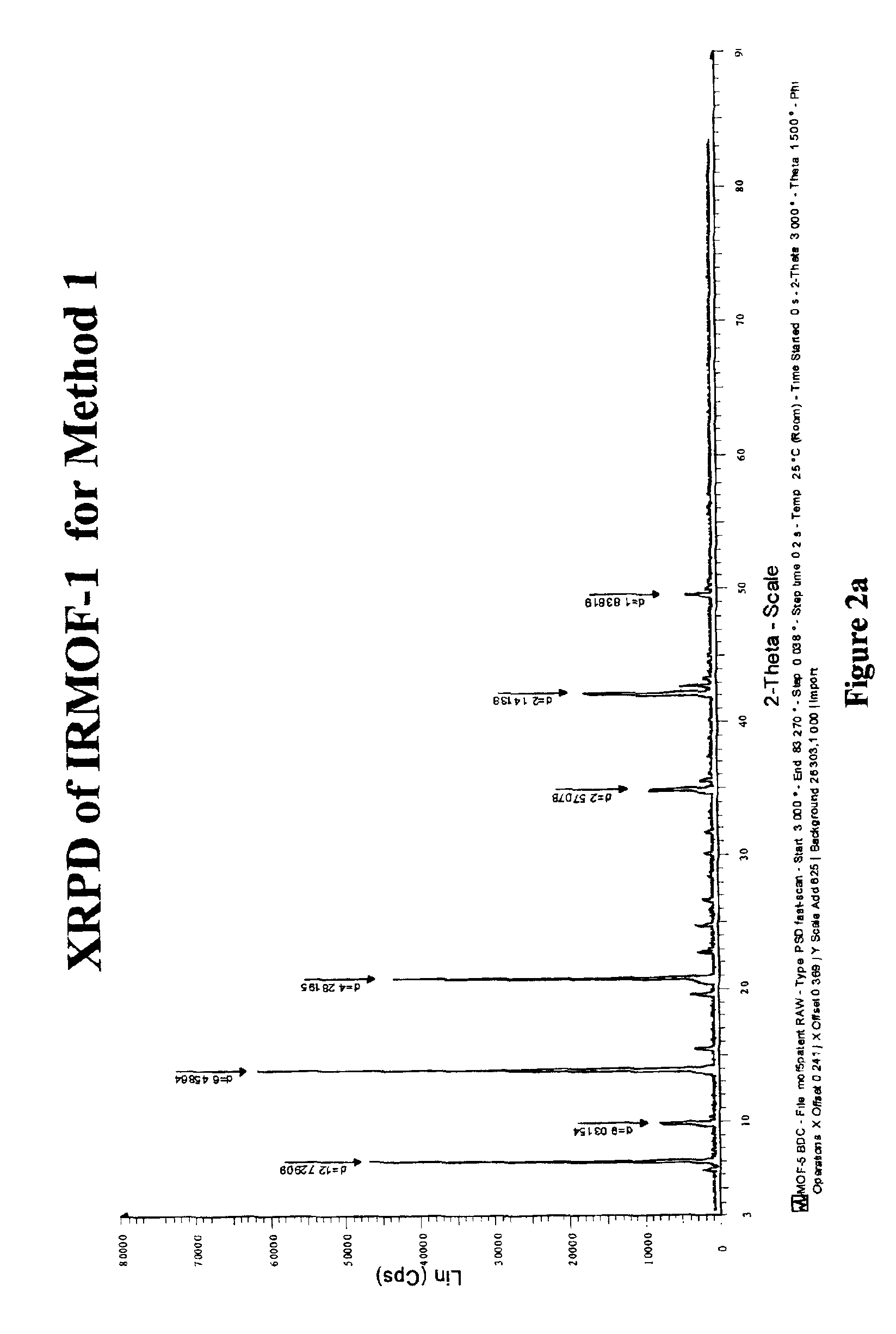
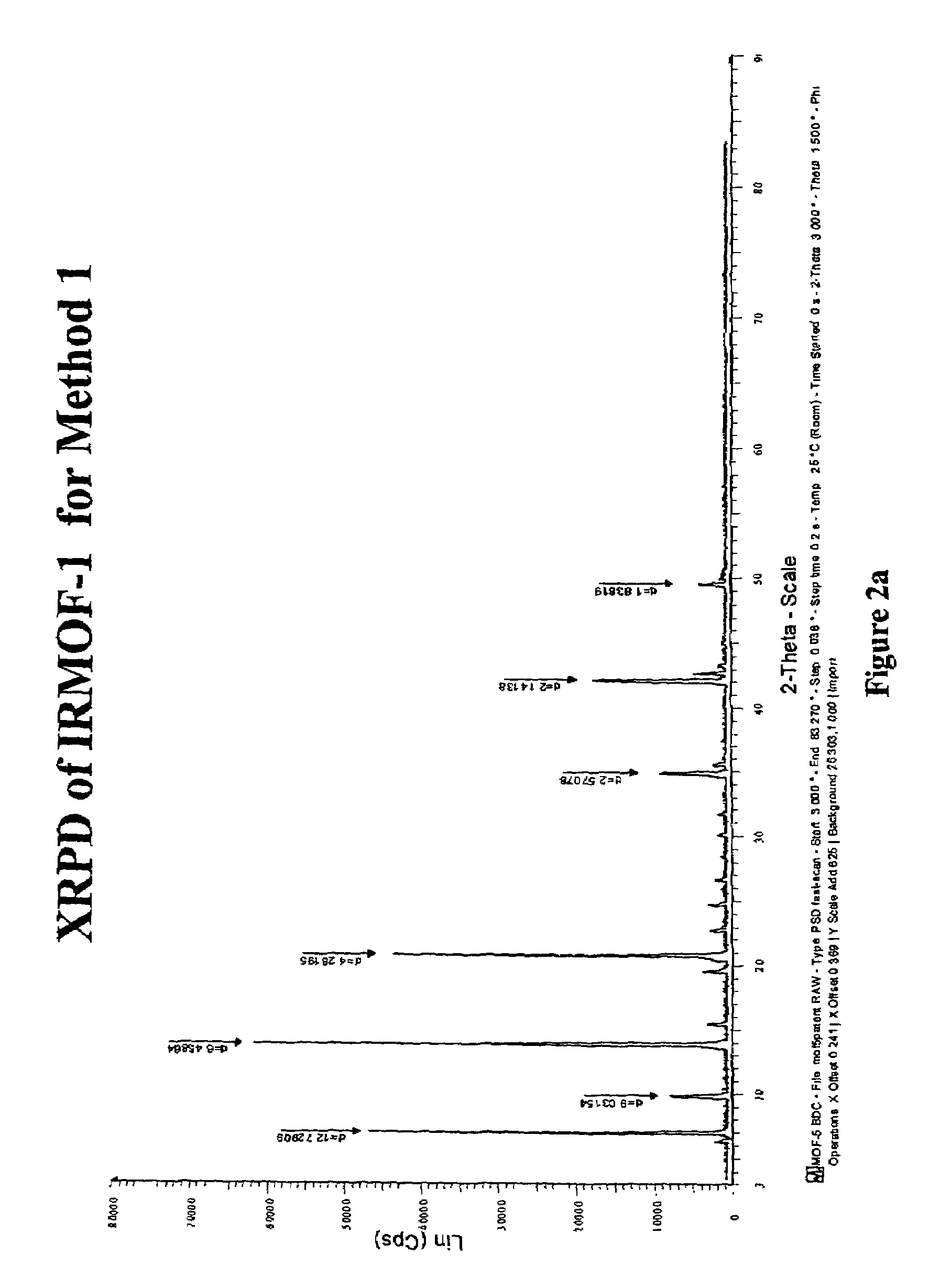
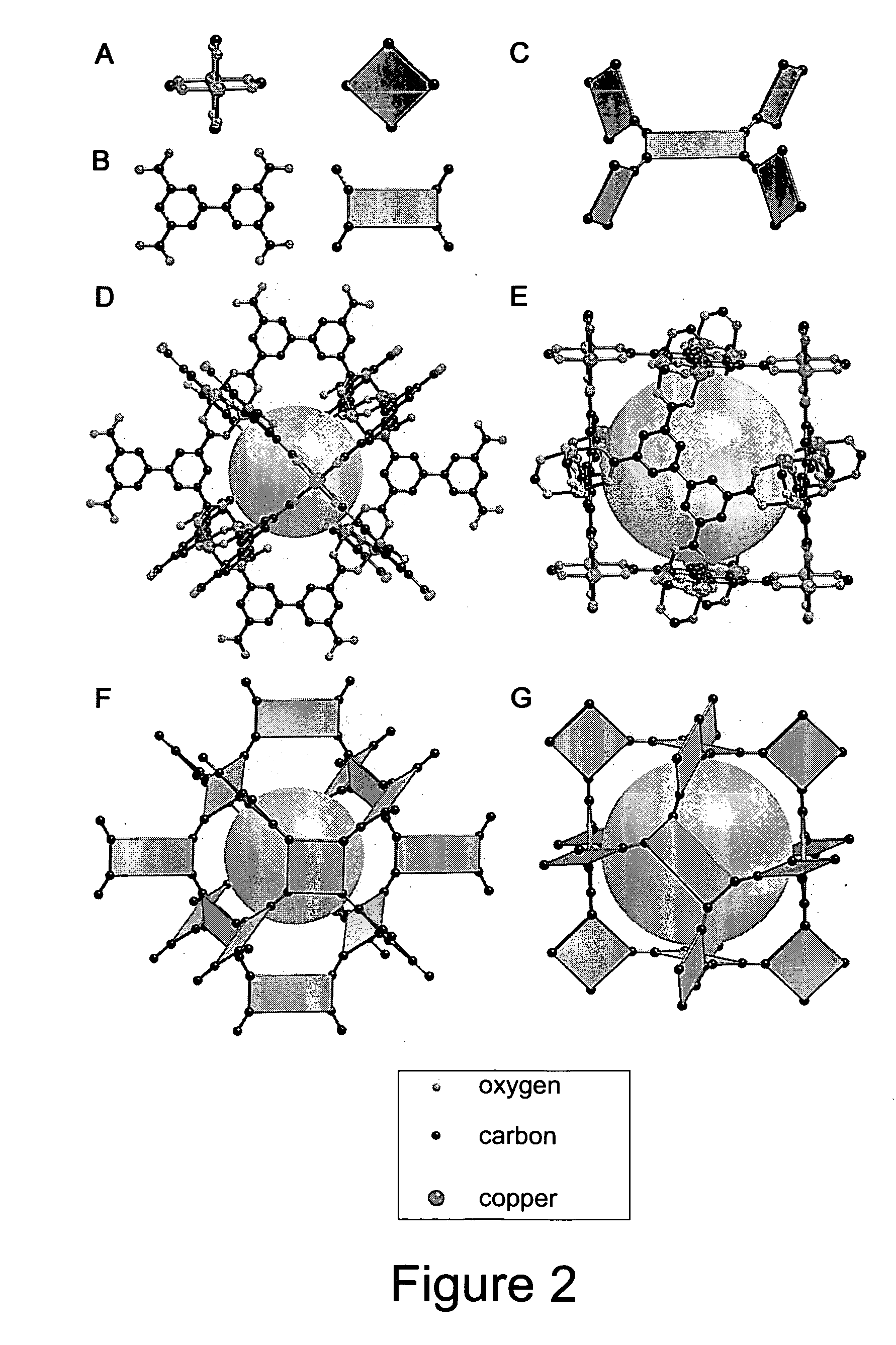
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS6930193B2High methane storage capacityIncrease storage capacityGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsOrganic linkingSystems design
An isoreticular metal-organic framework (IRMOF) and method for systematically forming the same. The method comprises the steps of dissolving at least one source of metal cations and at least one organic linking compound in a solvent to form a solution; and crystallizing the solution under predetermined conditions to form a predetermined IRMOF. At least one of functionality, dimension, pore size and free volume of the IRMOF is substantially determined by the organic linking compound.
Owner:RGT UNIV OF MICHIGAN
Energy efficient gas separation for fuel cells
InactiveUS20020142208A1Improve efficiencyReduce the ratioFuel cell heat exchangeFused electrolyte fuel cellsEngineeringDelivery system
An electrical current generating system is disclosed that includes a fuel cell operating at a temperature of at least about 250° C. (for example, a molten carbonate fuel cell or a solid oxide fuel cell), a hydrogen gas separation system or oxygen gas delivery system that includes a compressor or pump, and a drive system for the compressor or pump that includes means for recovering energy from at least one of the hydrogen gas separation system, oxygen gas delivery system, or heat of the fuel cell. The drive system could be a gas turbine system. The hydrogen gas separation system or the oxygen gas delivery system may include a pressure swing adsorption module.
Owner:AIR PROD & CHEM INC
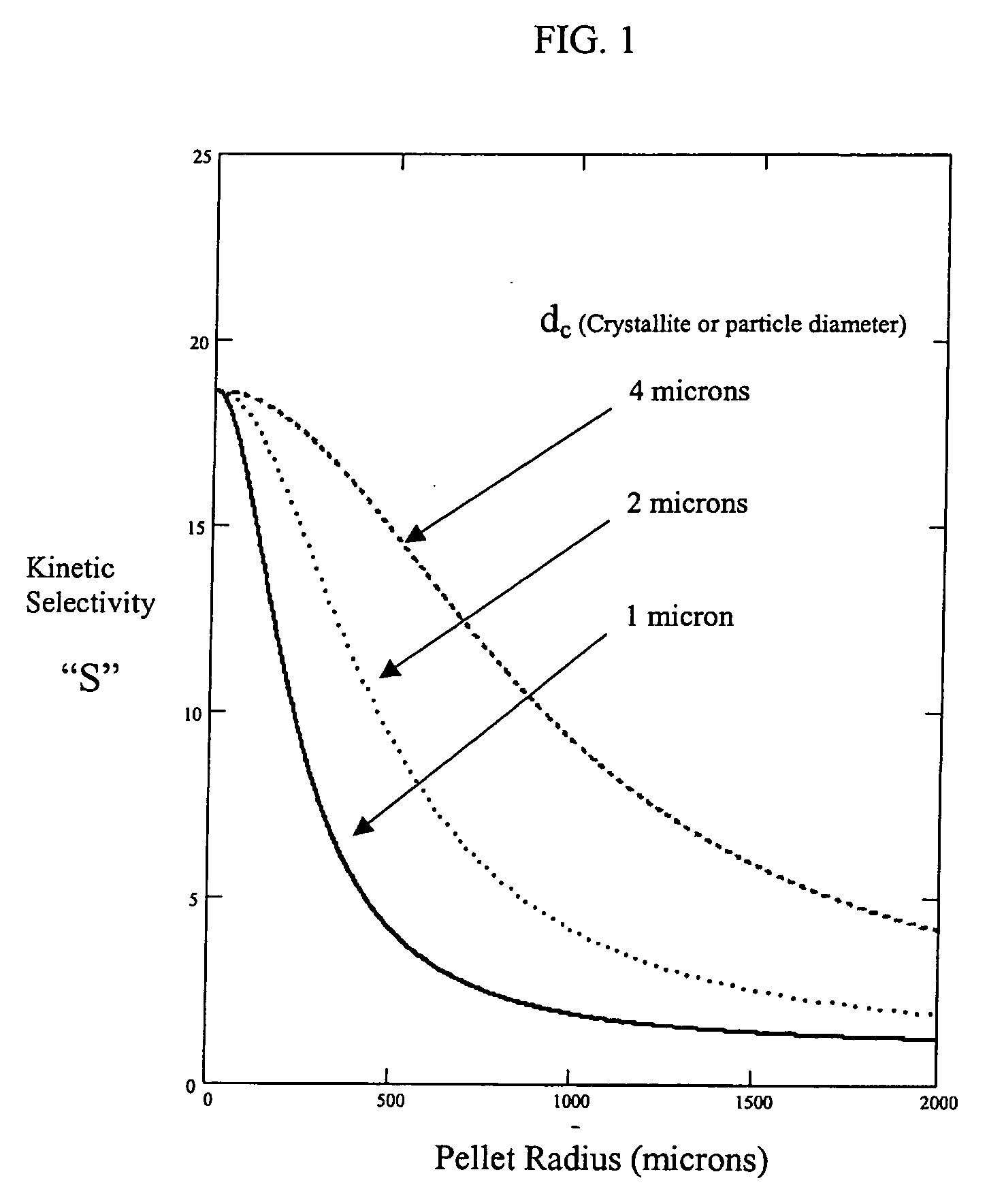
Engineered adsorbent structures for kinetic separation
ActiveUS20060169142A1Easy to controlHigh selectivityGas treatmentMethane captureProduction rateSorbent
Improved adsorbent sheet based parallel passage adsorbent structures for enhancing the kinetic selectivity of certain kinetic-controlled adsorption processes, such as PSA, TSA and PPSA processes, and combinations thereof, are provided. The enhancements in kinetic selectivity made possible through the implementation of the present inventive improved adsorbent structures may unexpectedly enable significant intensification of selected kinetic adsorption processes relative to attainable performance with conventional adsorbent materials in beaded or extruded form. Such process intensification enabled by the present inventive adsorbent structures may provide for increased adsorption cycle frequencies, and increased gas flow velocities within the adsorbent beds, which may increase the productivity and / or recovery of a kinetic adsorption system incorporating the inventive adsorbent structures.
Owner:AIR PROD & CHEM INC
Process for removing a target gas from a mixture of gases by swing adsorption
The present invention relates the separation of a target gas from a mixture of gases through the use of engineered structured adsorbent contactors in pressure swing adsorption and thermal swing adsorption processes. Preferably, the contactors contain engineered and substantially parallel flow channels wherein 20 volume percent or less of the open pore volume of the contactor, excluding the flow channels, is in the mesopore and macropore range.
Owner:EXXON RES & ENG CO
Nanometer hole metal-organic frame material in single-level or multilevel pore canal structure and preparation method thereof
The invention relates to a nanometer hole metal-organic frame material in a single-level or multilevel pore canal structure as well as a preparation method and the application thereof. The preparation method comprises the following steps: at least one metal salt and at least one polyfunctional group organic ligand reacts with at least one template agent in at least one solvent under the condition that no hole aid agent exists or only one hole aid agent exist, and single-level or multilevel nanometer holes or channels with the size of 0.5-100 nm exist in at least one direction in the internal space of the obtained metal-organic frame solid. The nanometer hole metal-organic frame material with large hole size and the nanometer hole metal-organic frame material in the layered multilevel pore canal structure can be obtained without the synthesis of the large-size organic ligand and have wide applications.
Owner:ANHUI UNIVERSITY
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
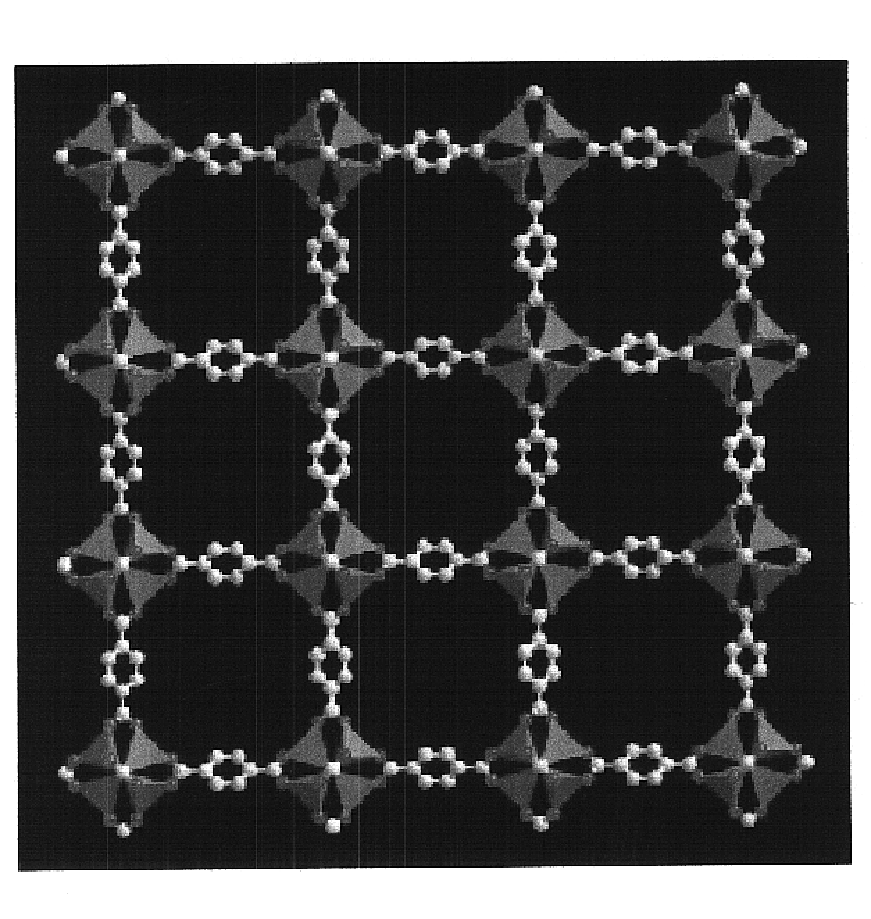
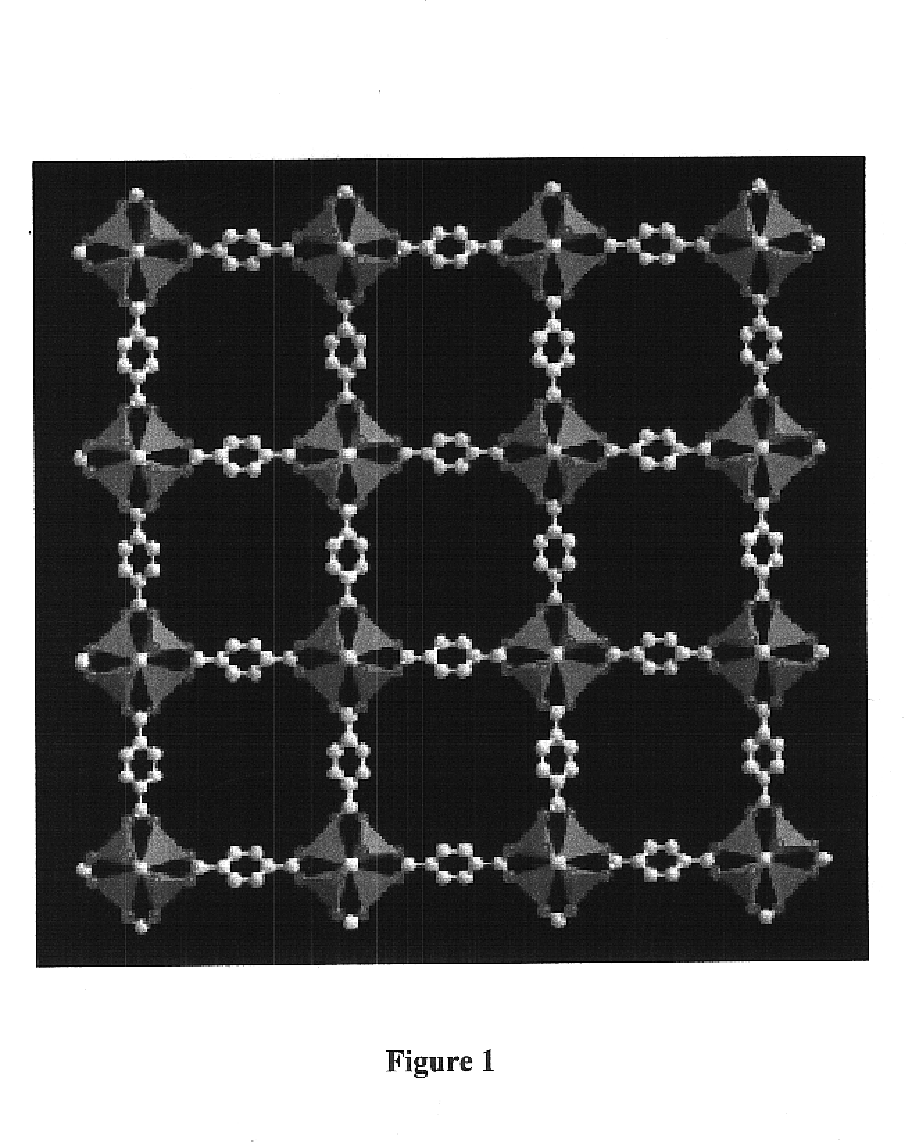
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
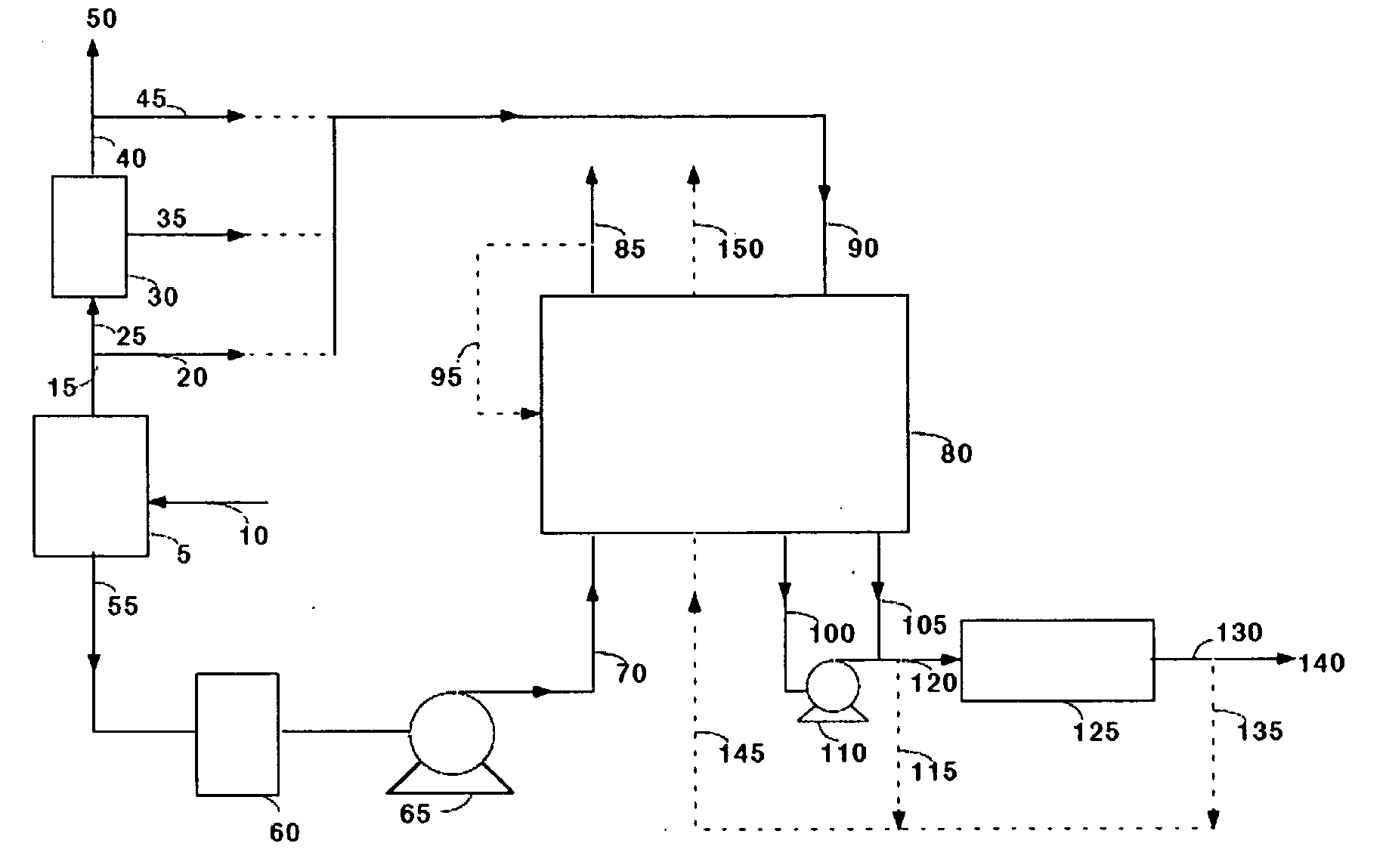
Carbon Dioxide Recovery
Disclosed herein is a method and system for separating carbon dioxide (CO2) from a CO2 containing gas stream containing water vapor and additional impurities, for example, nitrogen, oxygen, sulfur oxides, nitrogen oxides, and mercury. The CO2 is captured by subjecting the CO2 gas feed stream to a temperature swing adsorption step. The temperature swing adsorption step comprises an adsorption step for producing a substantially dry carbon dioxide-depleted stream, and an adsorbent regeneration step comprising heating the adsorbent bed to produce a substantially water vapor-free carbon dioxide stream. Moisture from the gas stream containing CO2 is optionally removed by pressure swing adsorption, temperature swing adsorption, membrane separation, or absorption prior to CO2 capture.
Owner:INNOSEPRA LLC
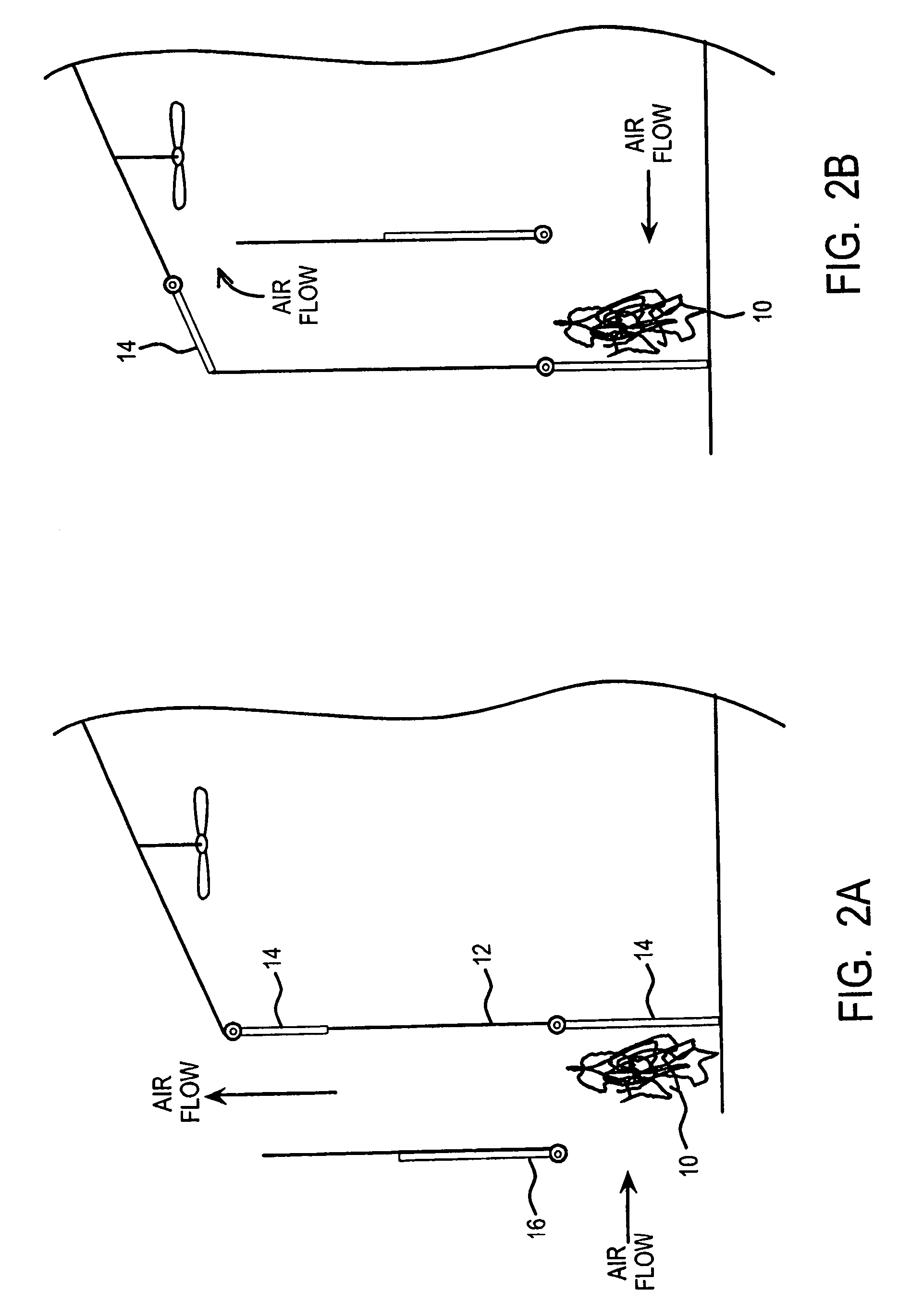
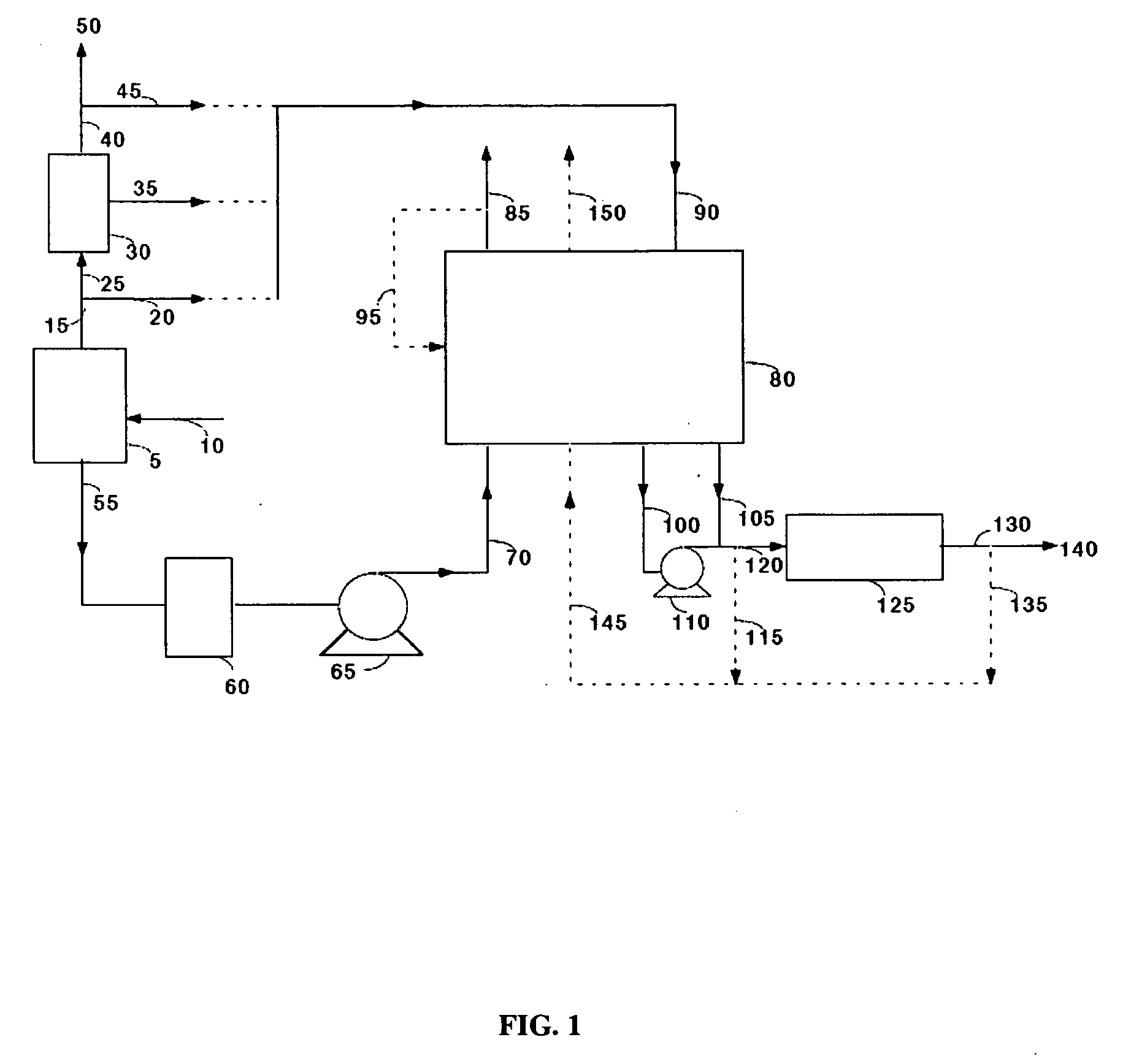
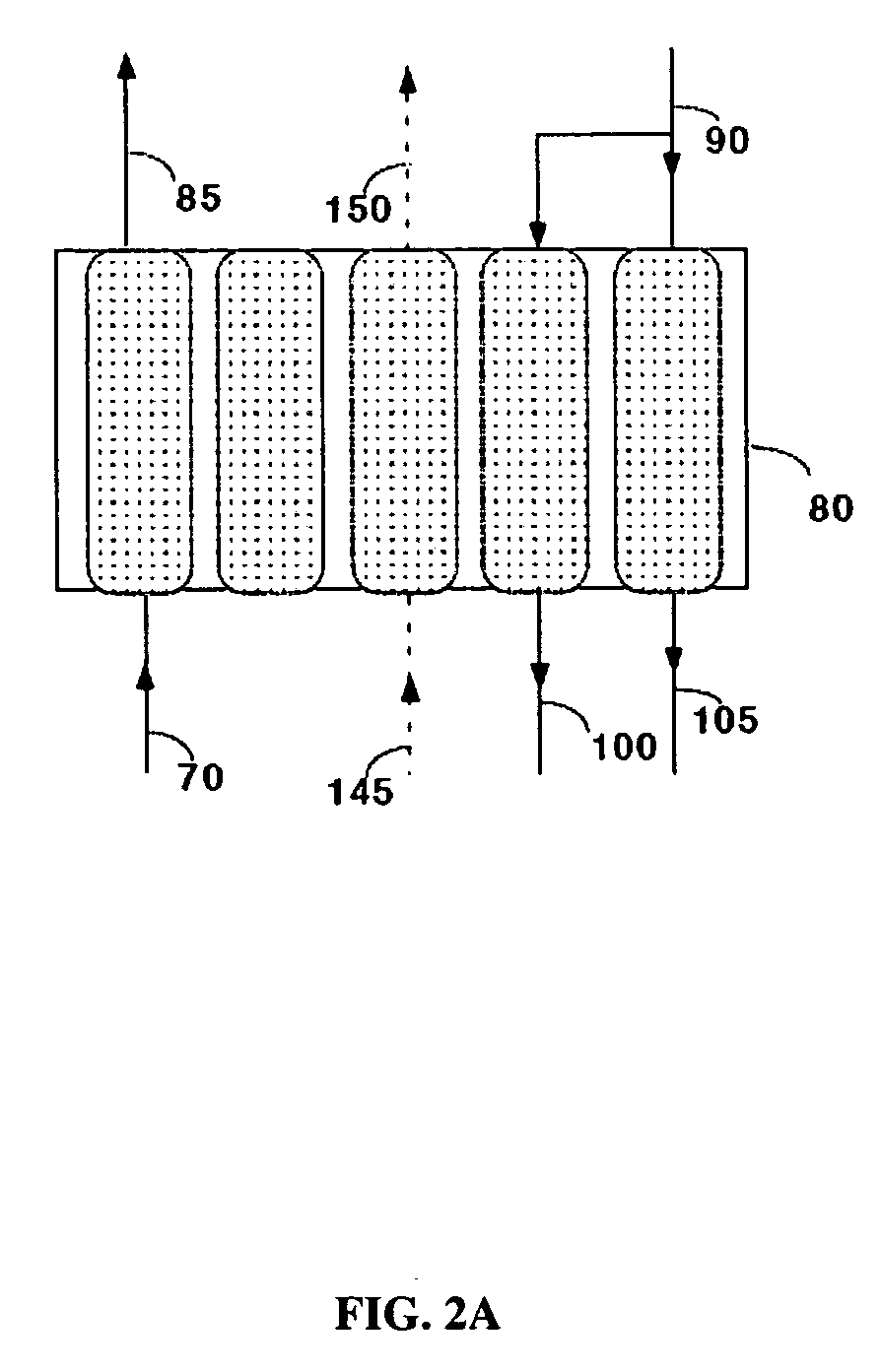
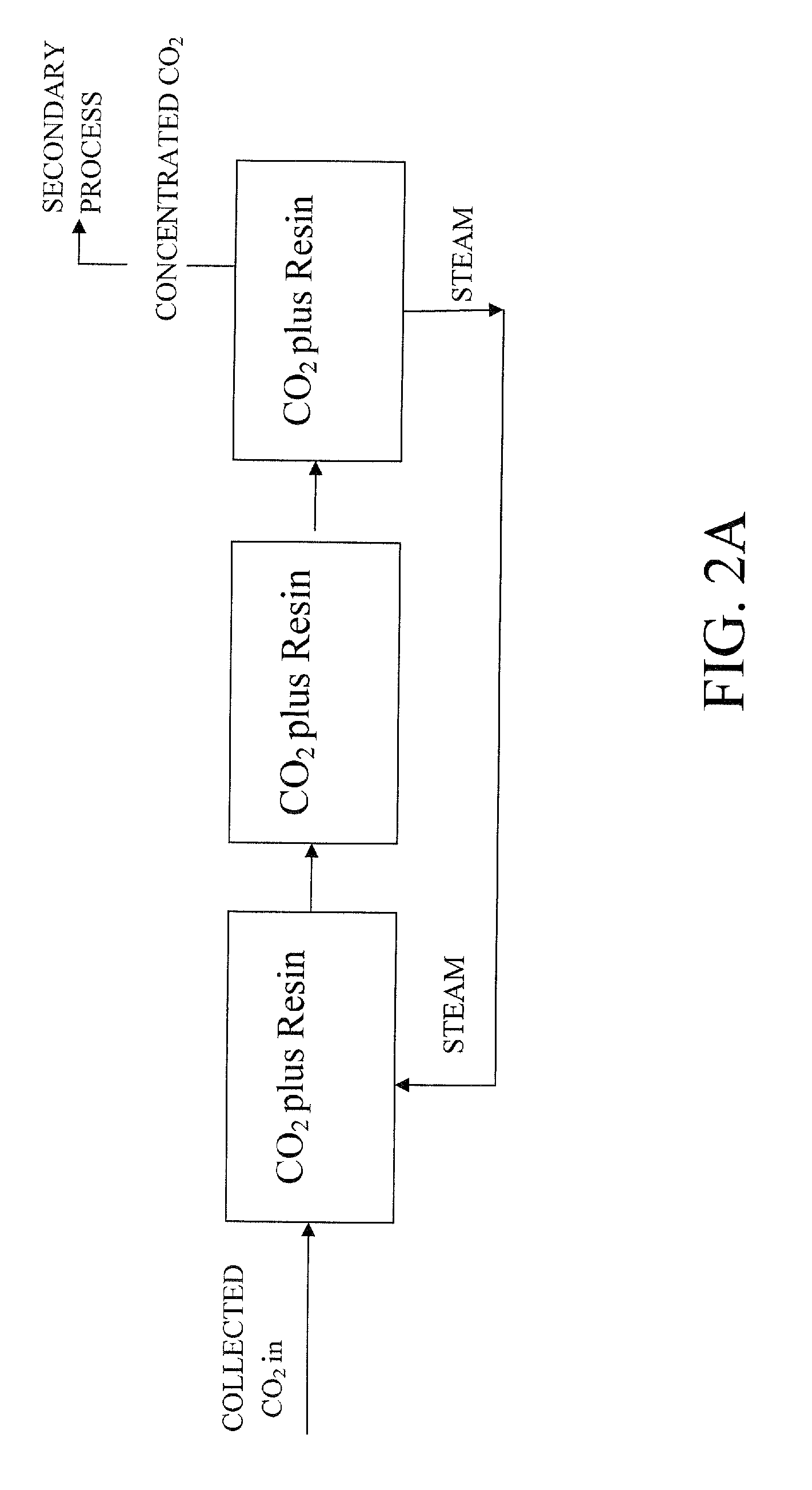
Extraction and sequestration of carbon dioxide
The present disclosure provides a method and apparatus for extracting carbon dioxide (CO2) from a fluid stream and for delivering that extracted CO2 to controlled environments for utilization by a secondary process. Various extraction and delivery methods are disclosed specific to certain secondary uses, included the attraction of CO2-sensitive insects, the ripening and preservation of produce, and the neutralization of brine.
Owner:KILIMANJARO ENERGY
Separation of carbon dioxide (CO2) from gas mixtures
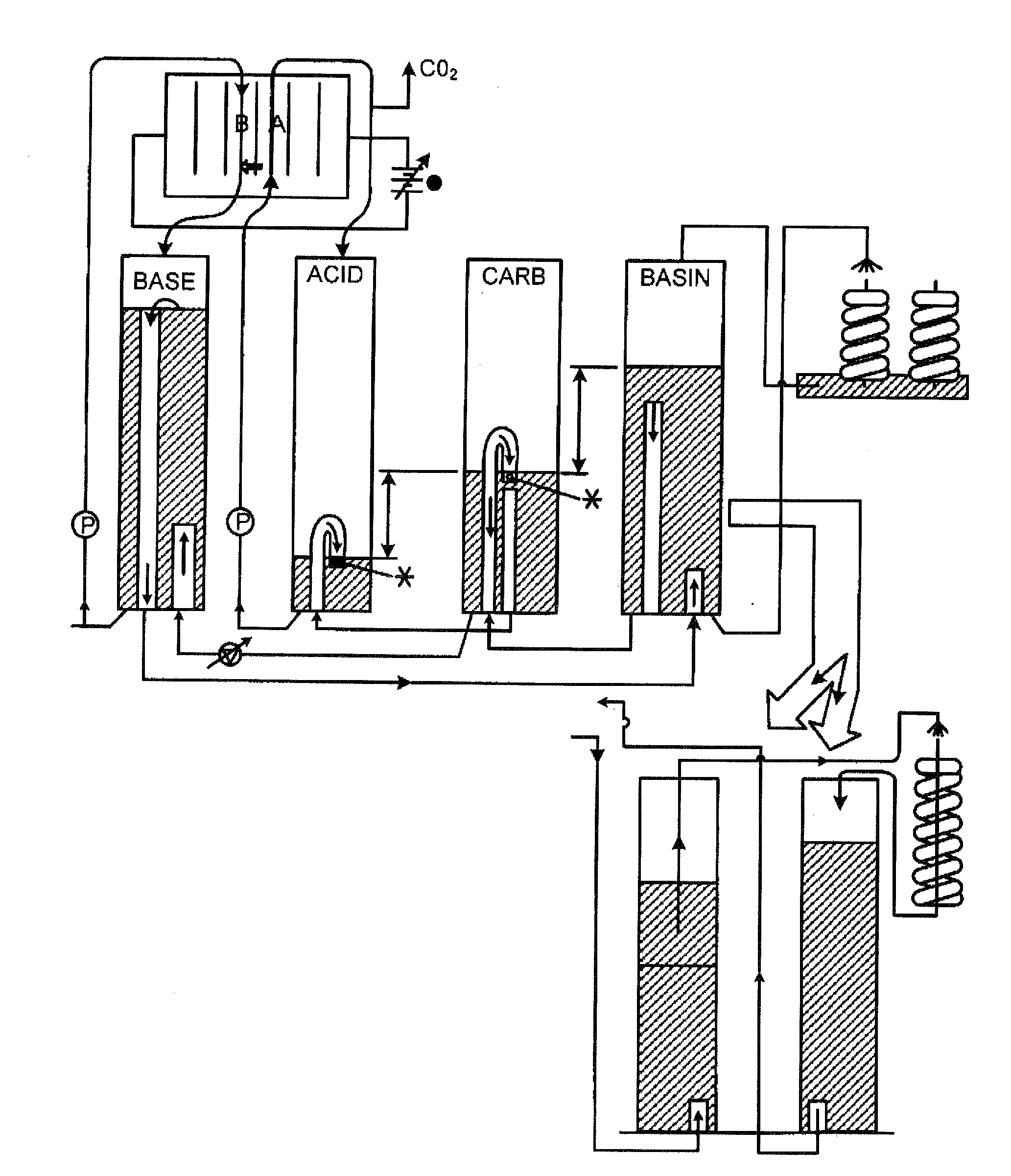
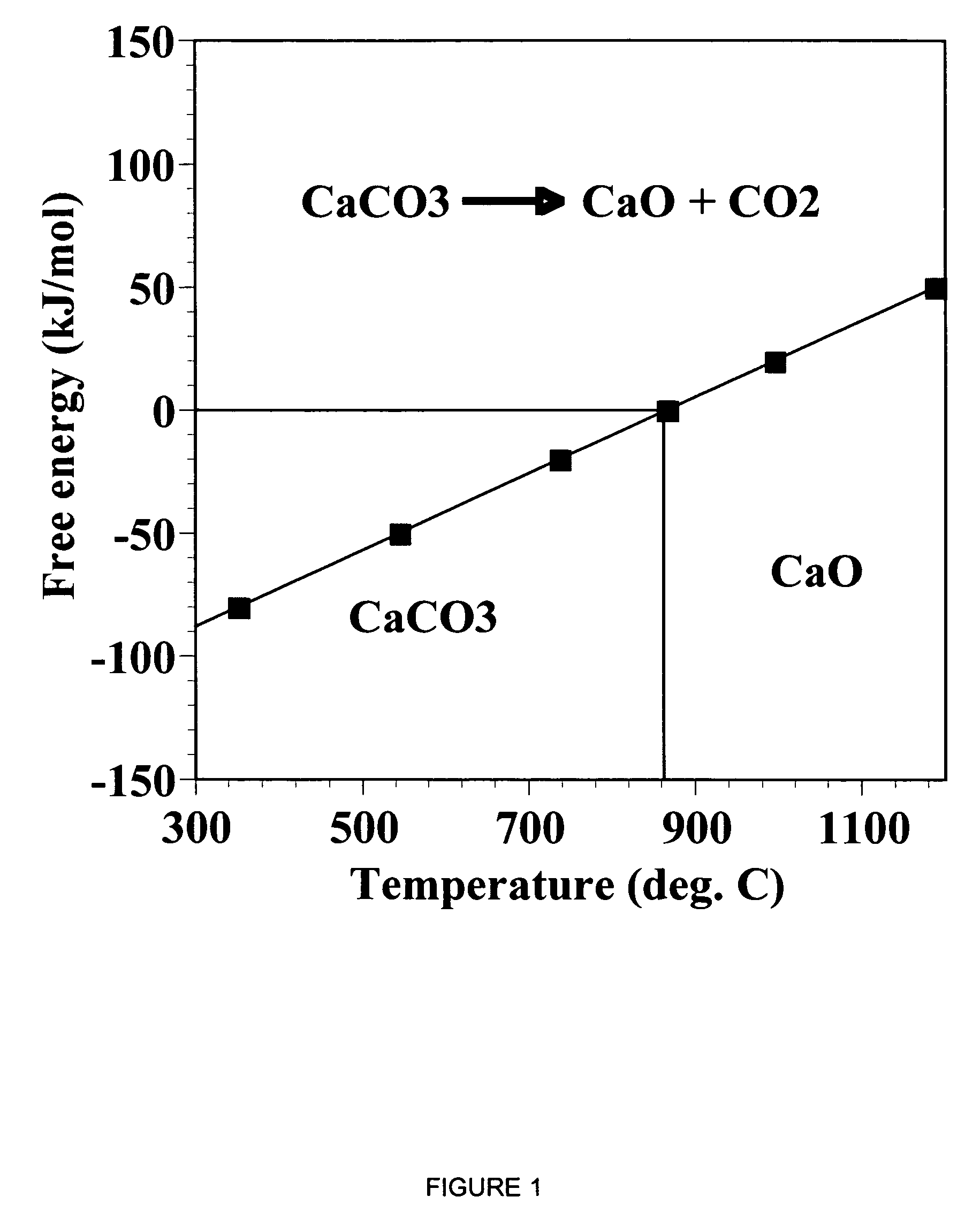
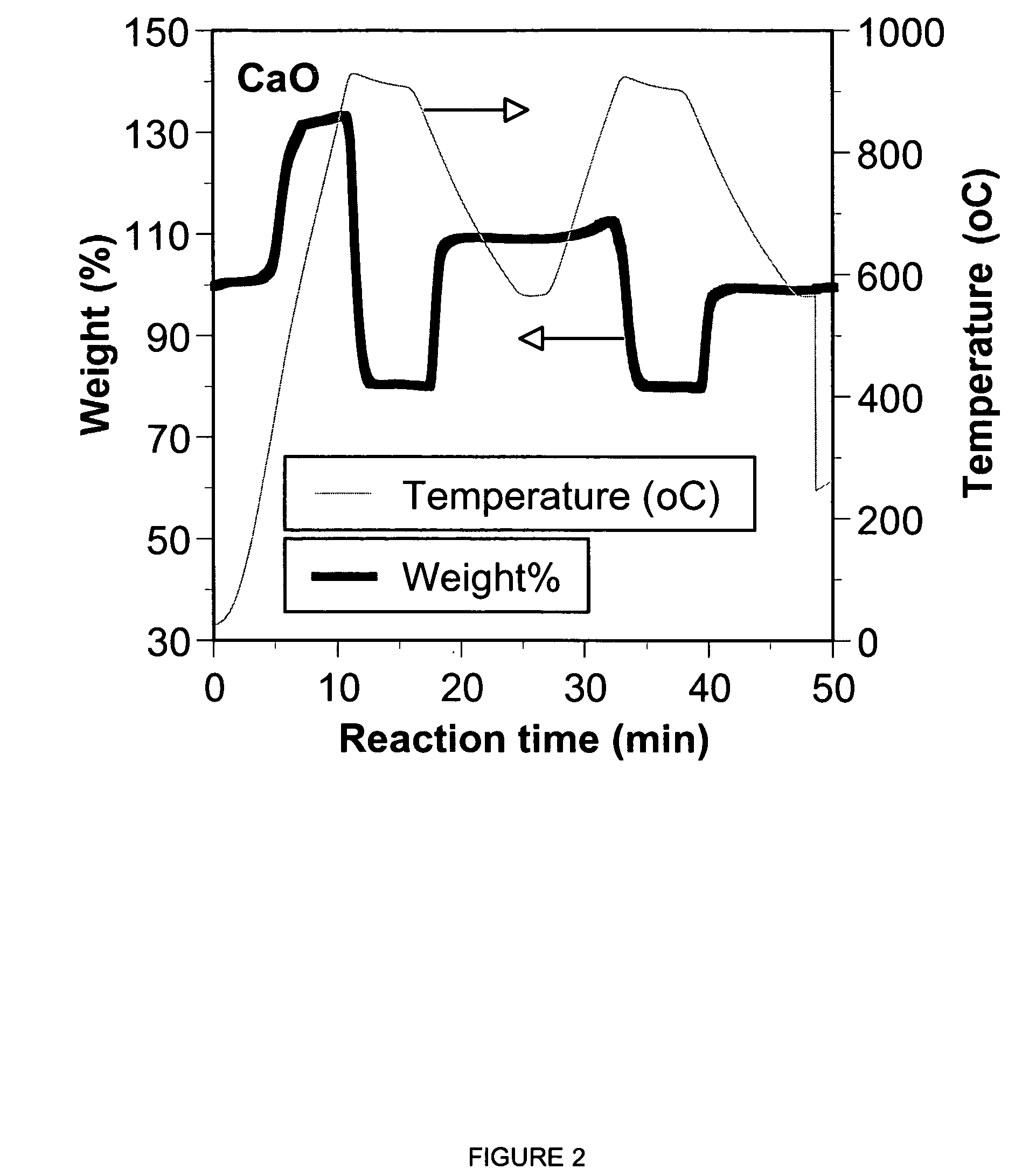
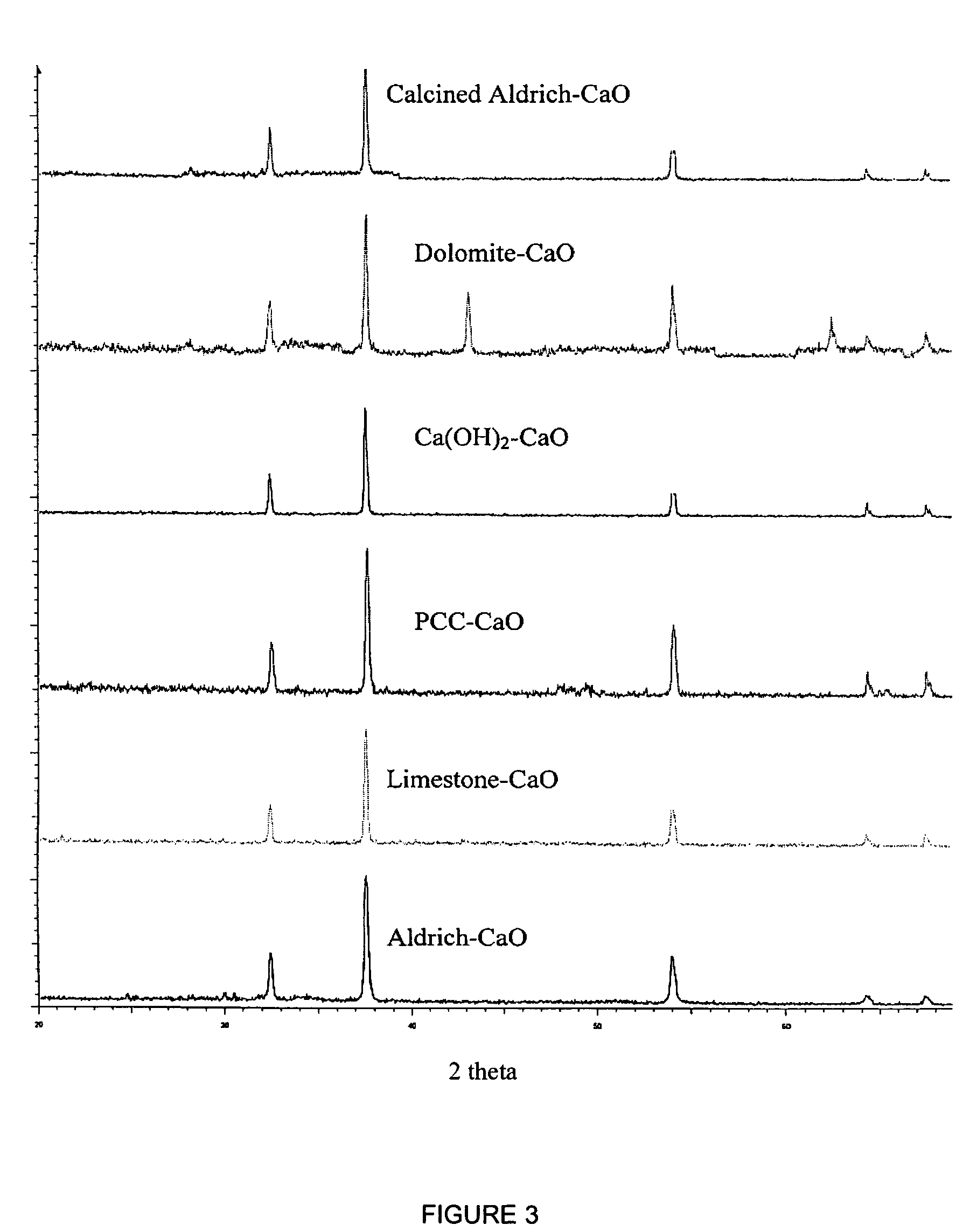
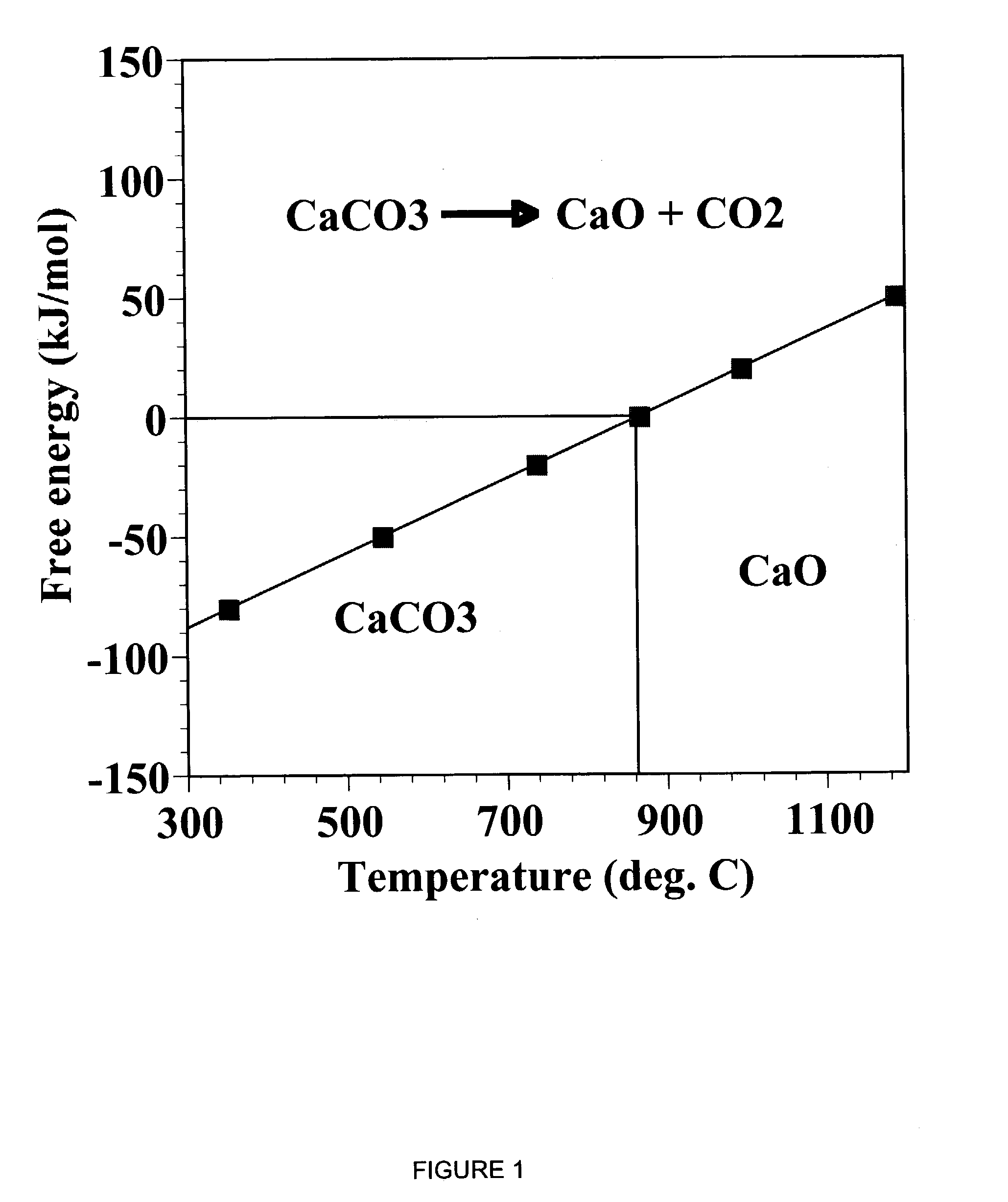
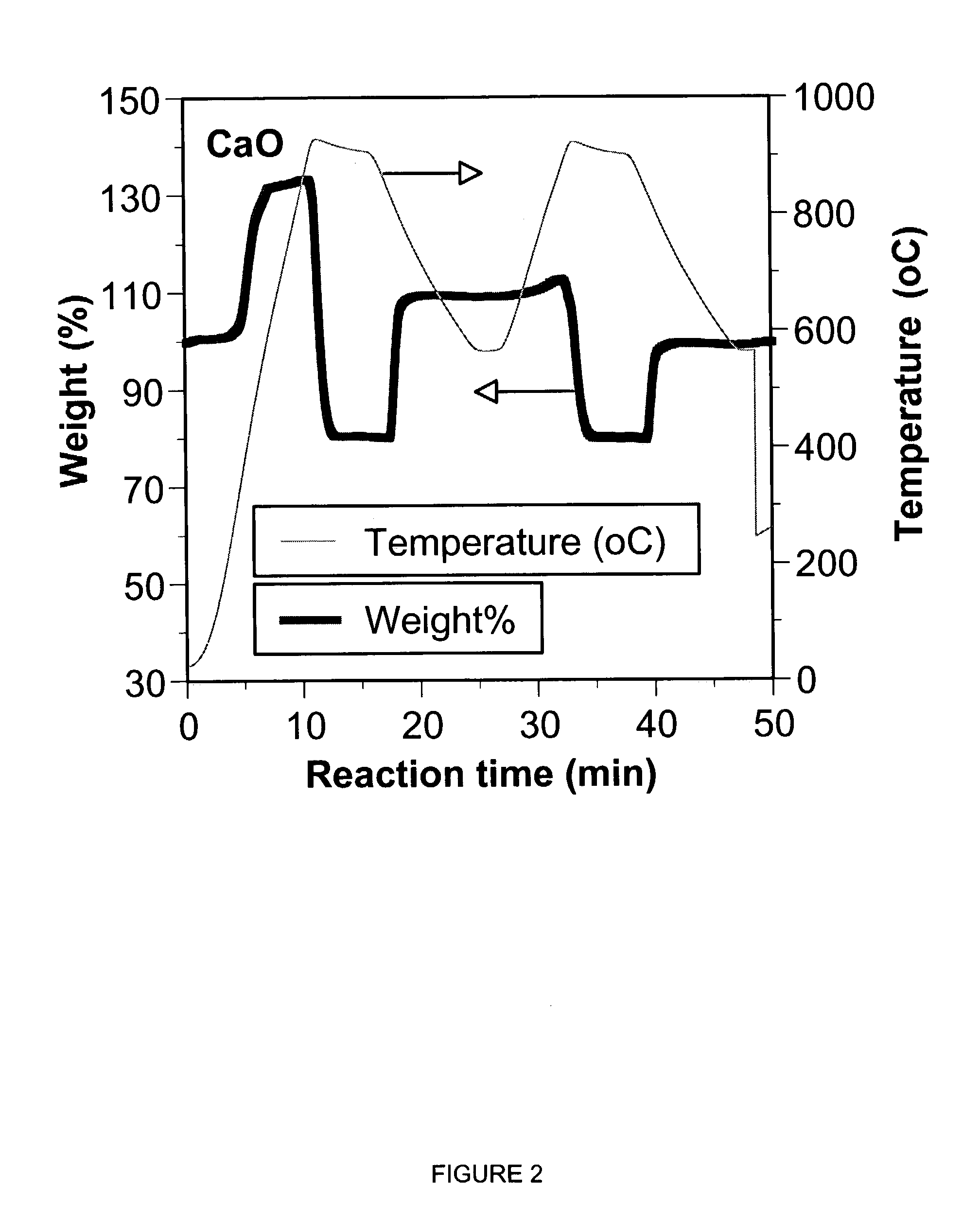
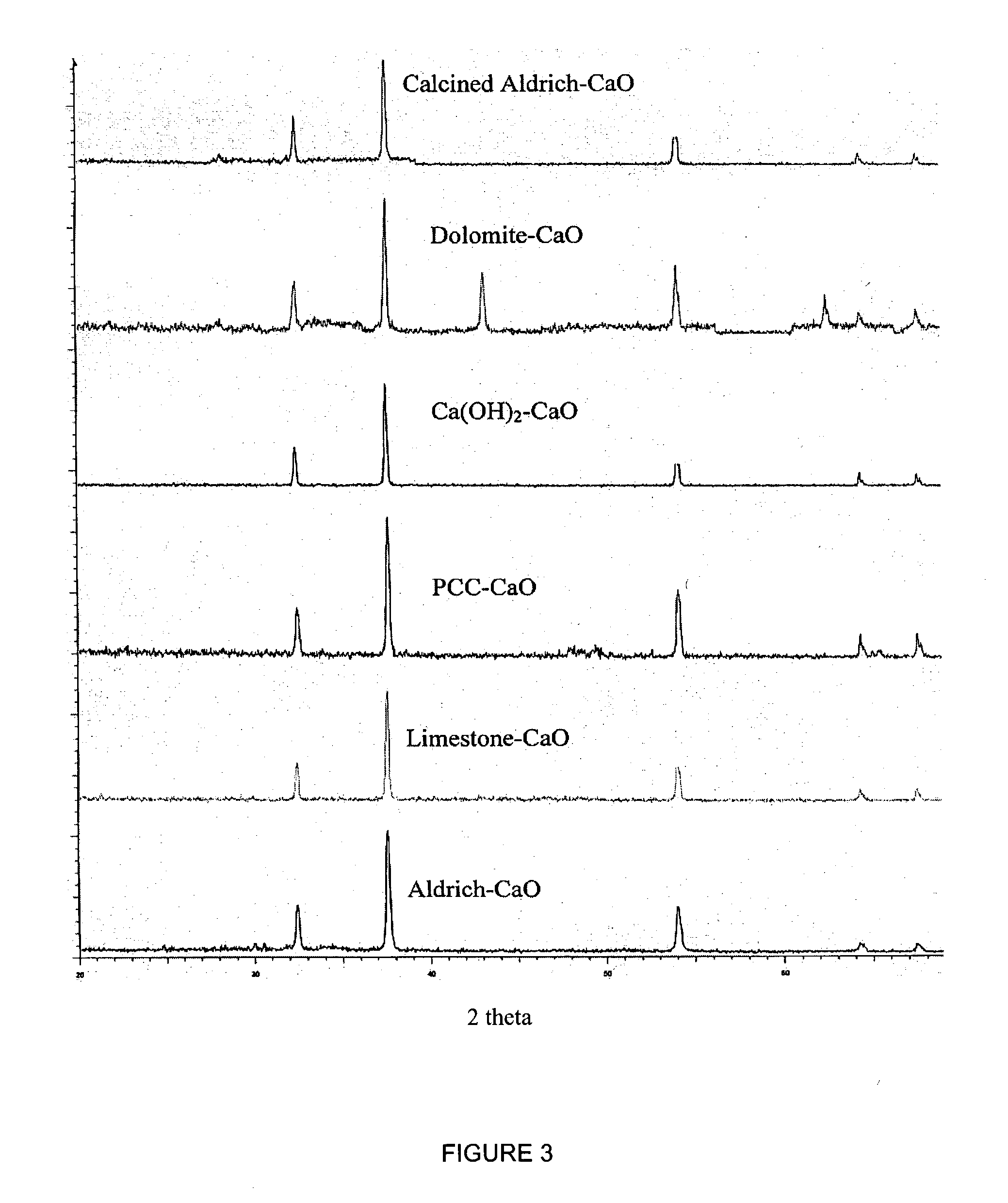
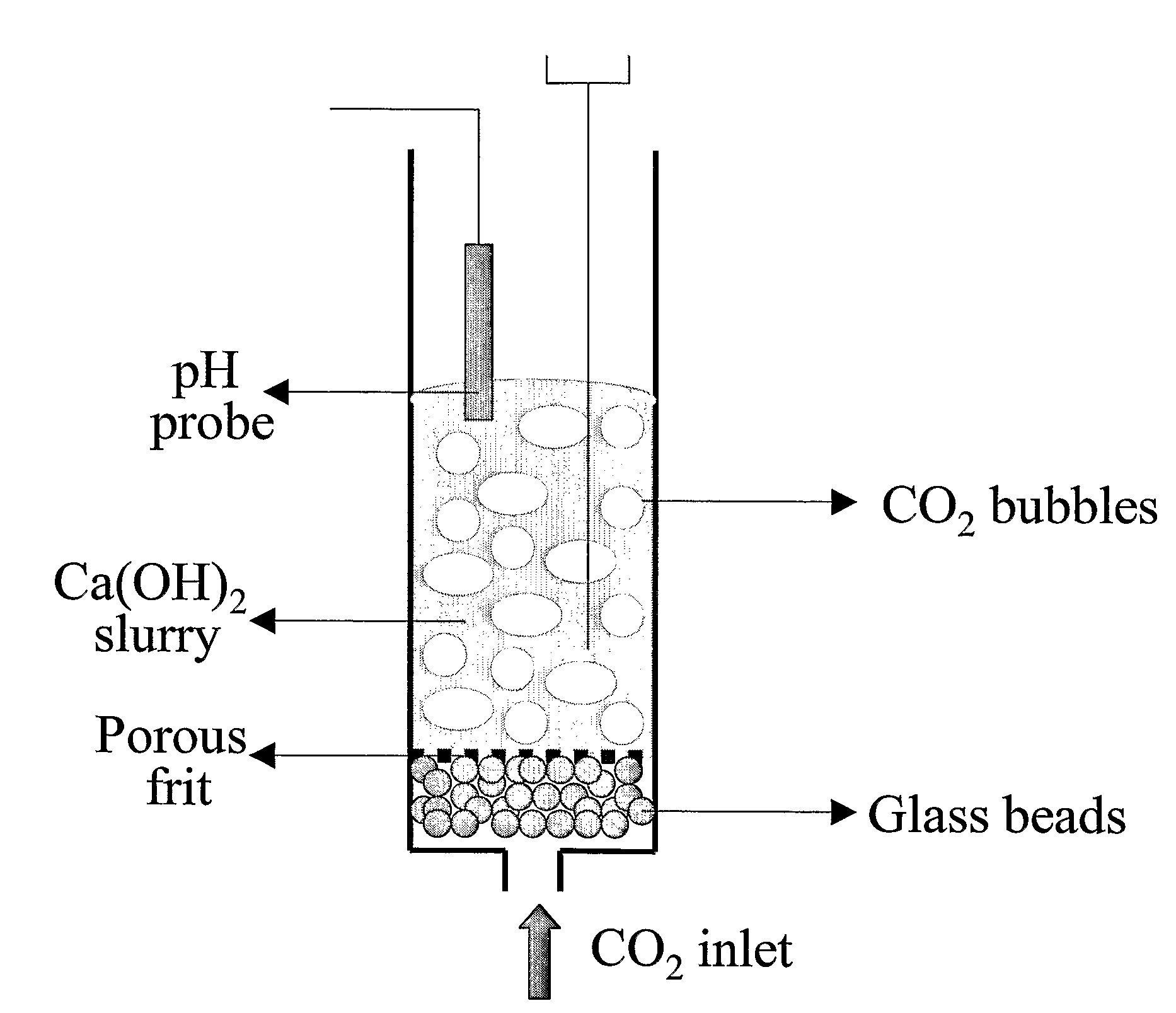
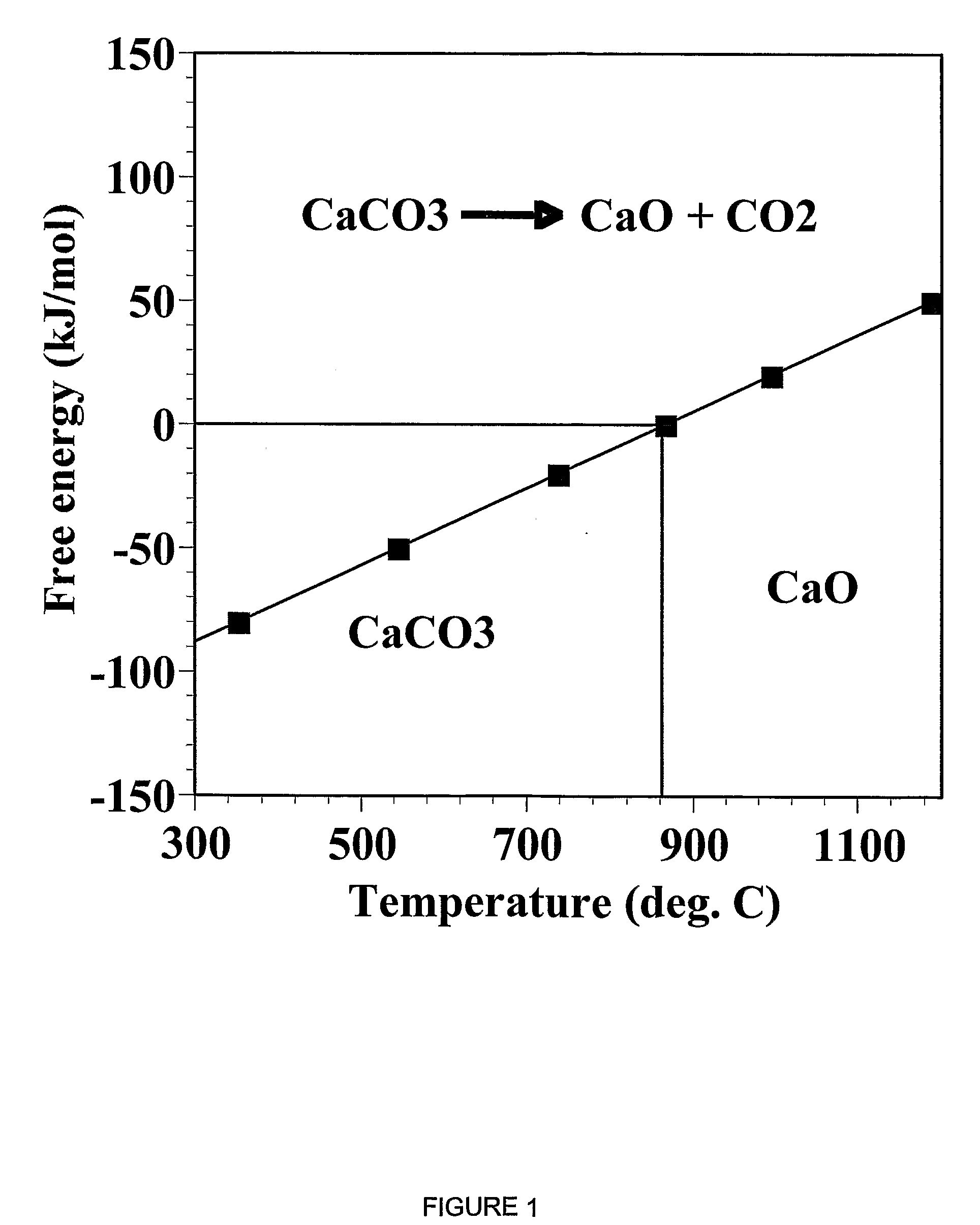
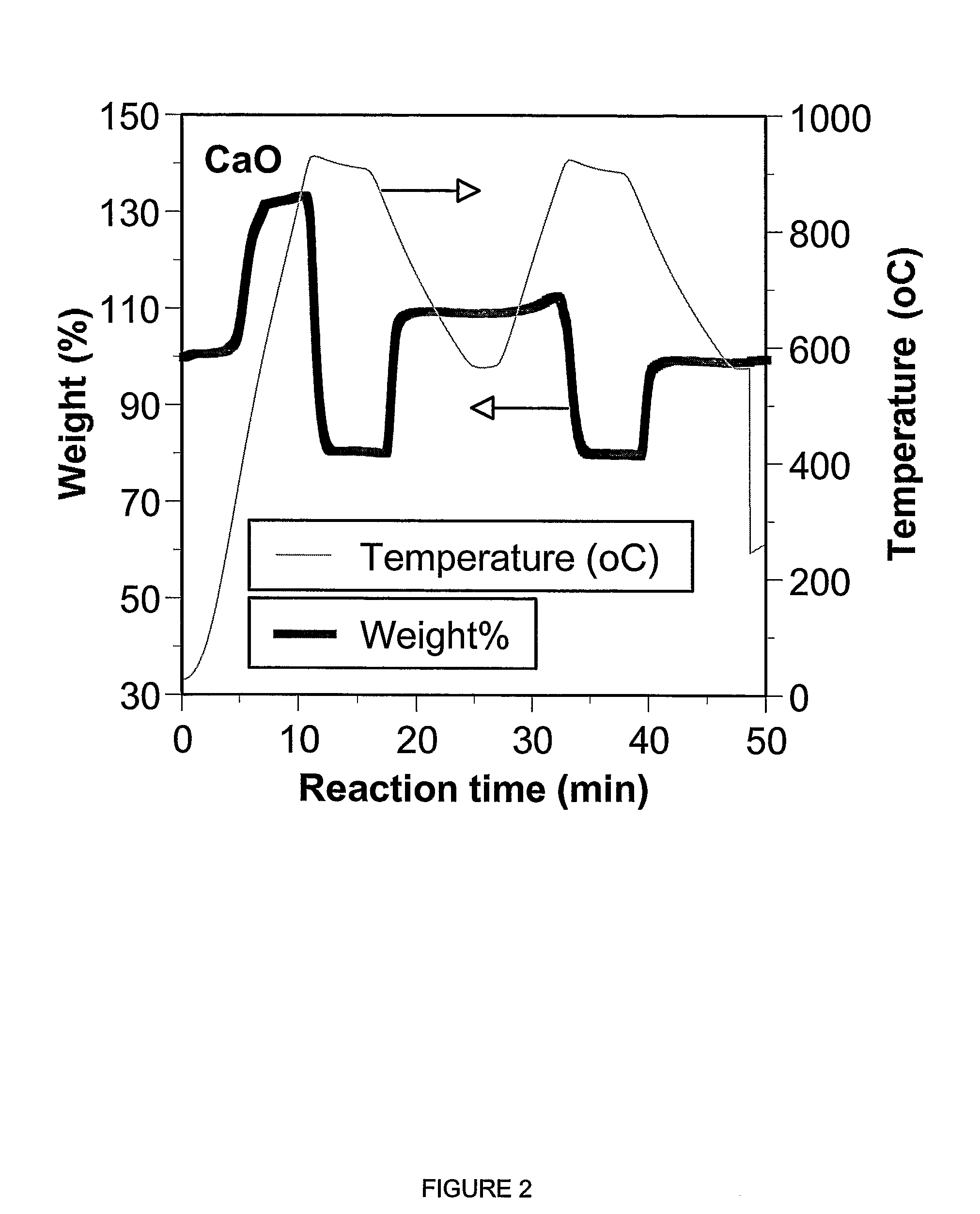
ActiveUS7618606B2Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentCo2 removalSorbent
A reaction-based process has been developed for the selective removal of carbon dioxide from a multicomponent gas mixture. The proposed process effects the separation of CO2 from a mixture of gases by its reaction with metal oxides. The Calcium based Reaction Separation for CO2 process consists of contacting a CO2 laden gas with calcium oxide in a reactor such that CaO captures the CO2 by the formation of calcium carbonate. Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent. The “regenerated” CaO is then recycled for the further capture of more CO2. This process also identifies the application of a mesoporous CaCO3 structure, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
High gas adsorption in a microporous metal-organic framework with open-metal sites
A gas storage material contains a metal-organic framework that includes a plurality of metal clusters and a plurality of charged multidentate linking ligands that connect adjacent metal clusters. Each metal cluster includes one or more metal ions and at least one open metal site. The metal-organic framework includes one or more sites for storing molecular hydrogen. A hydrogen storage system using the hydrogen storage material is provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
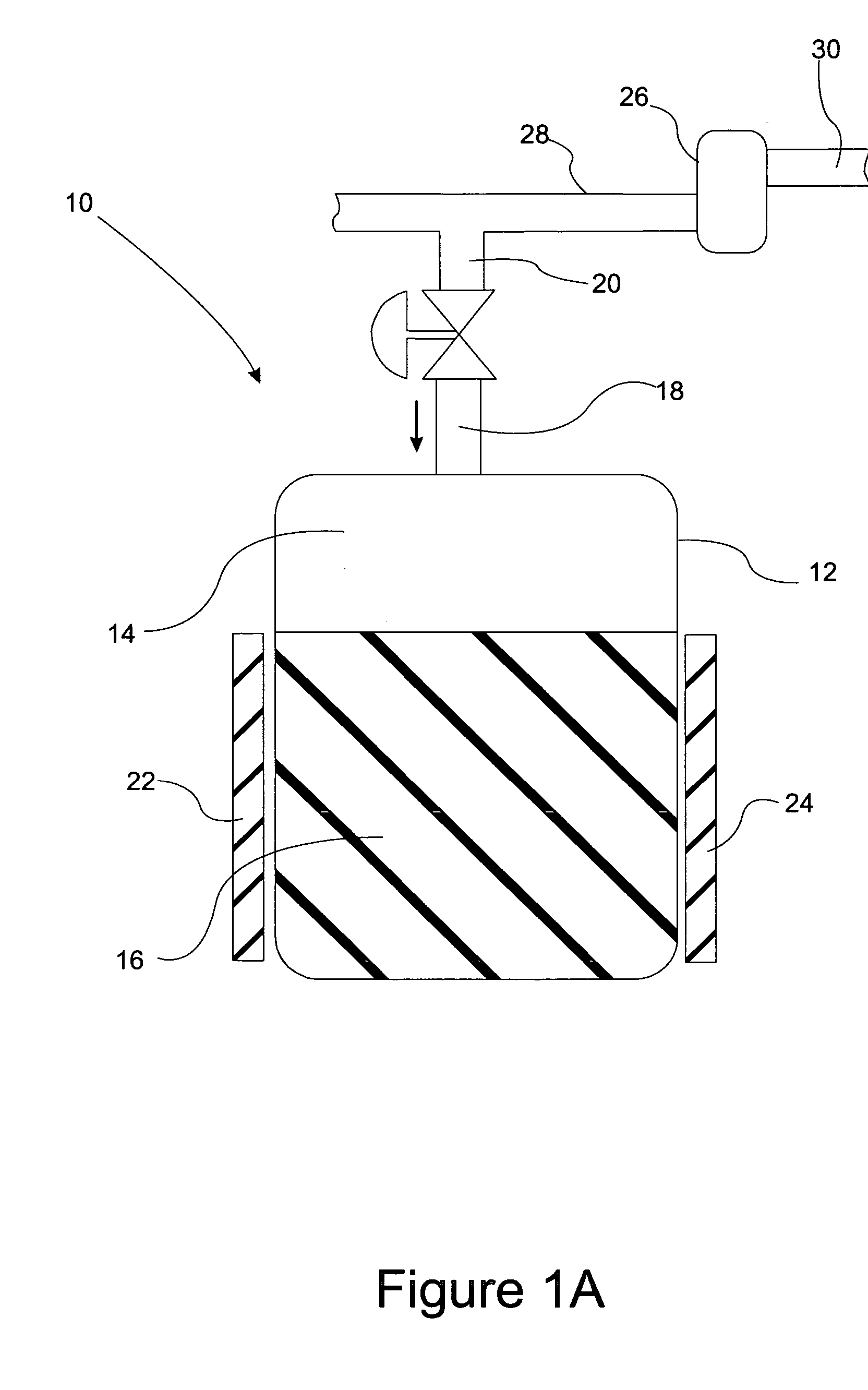
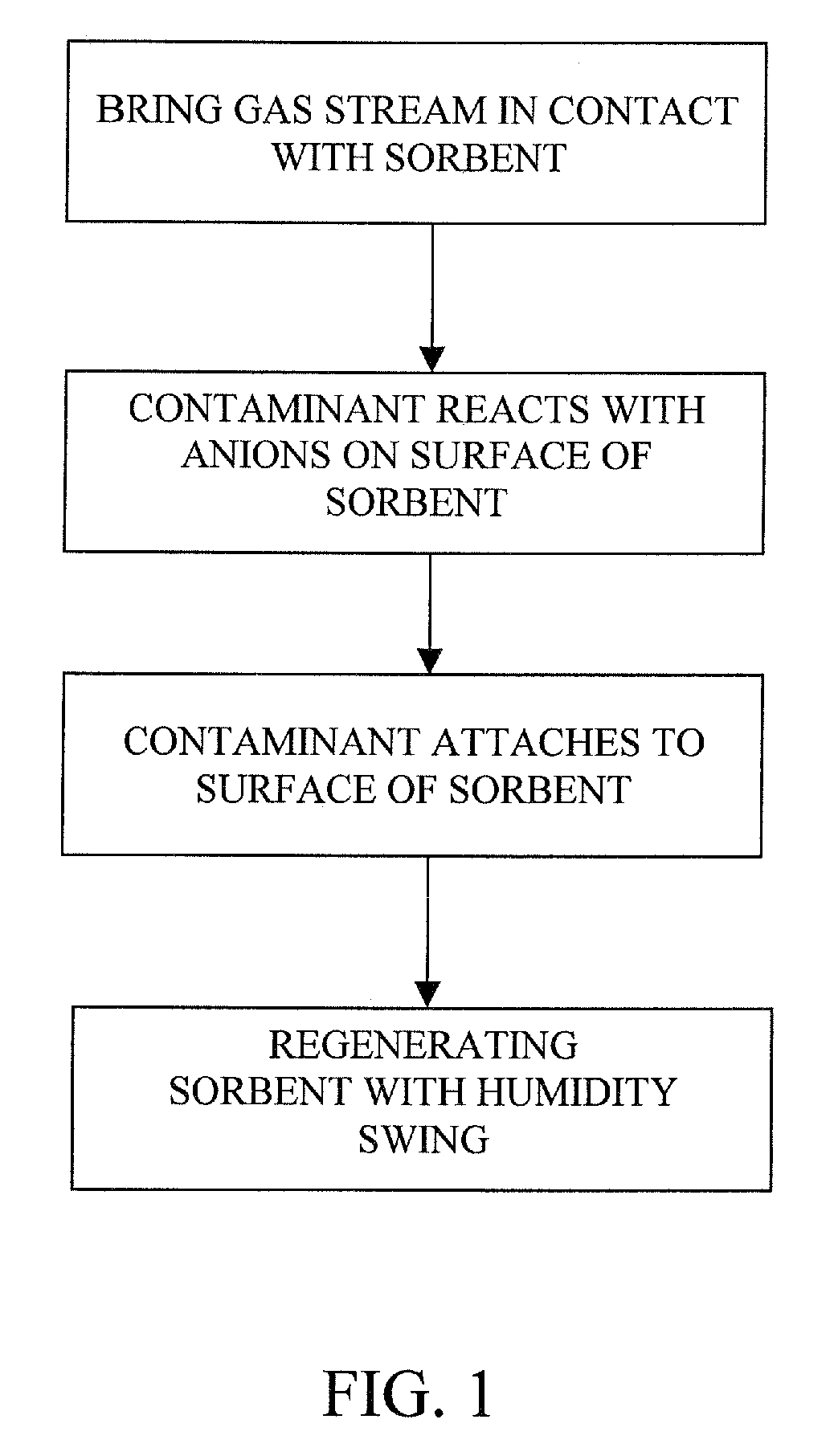
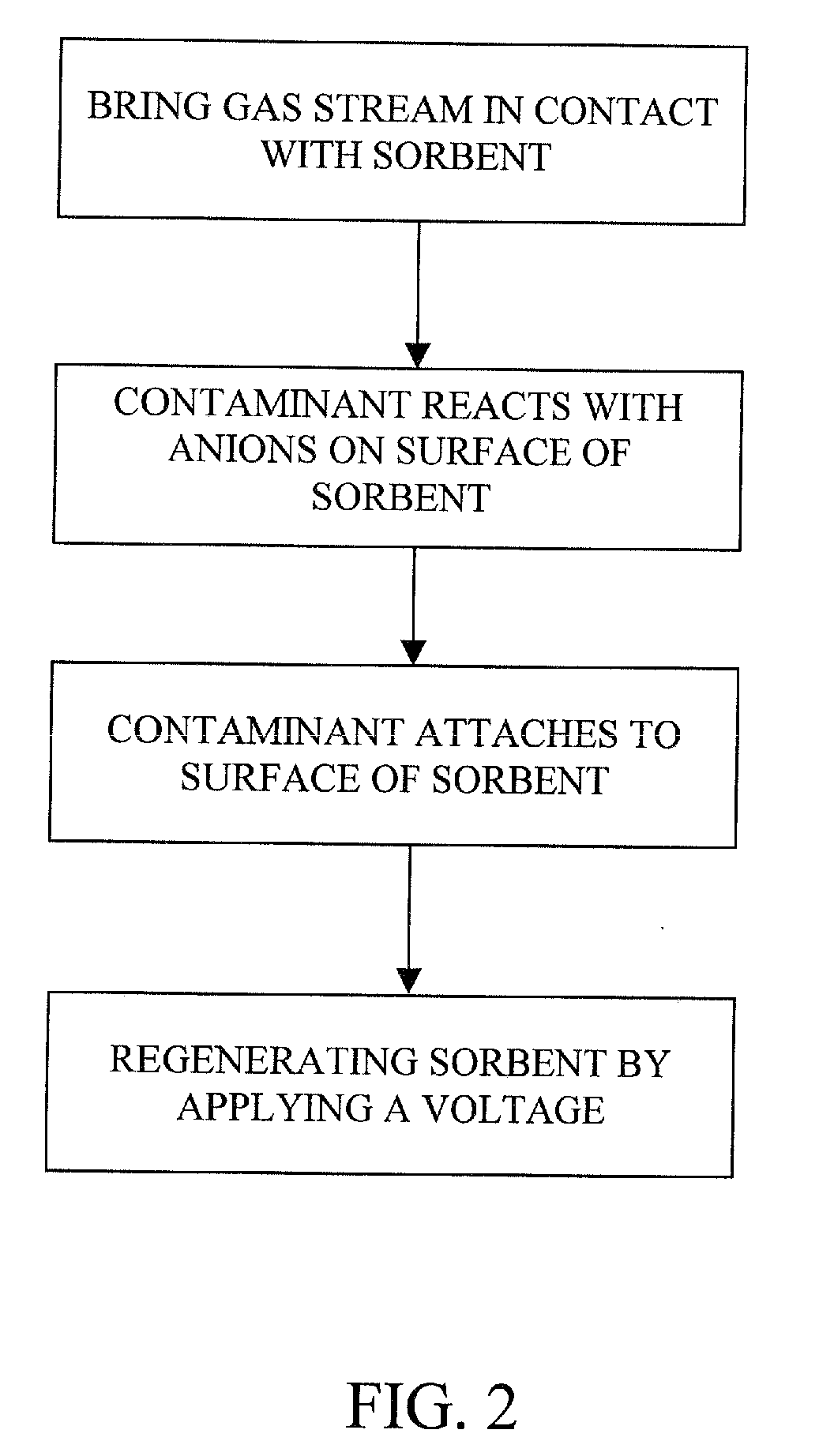
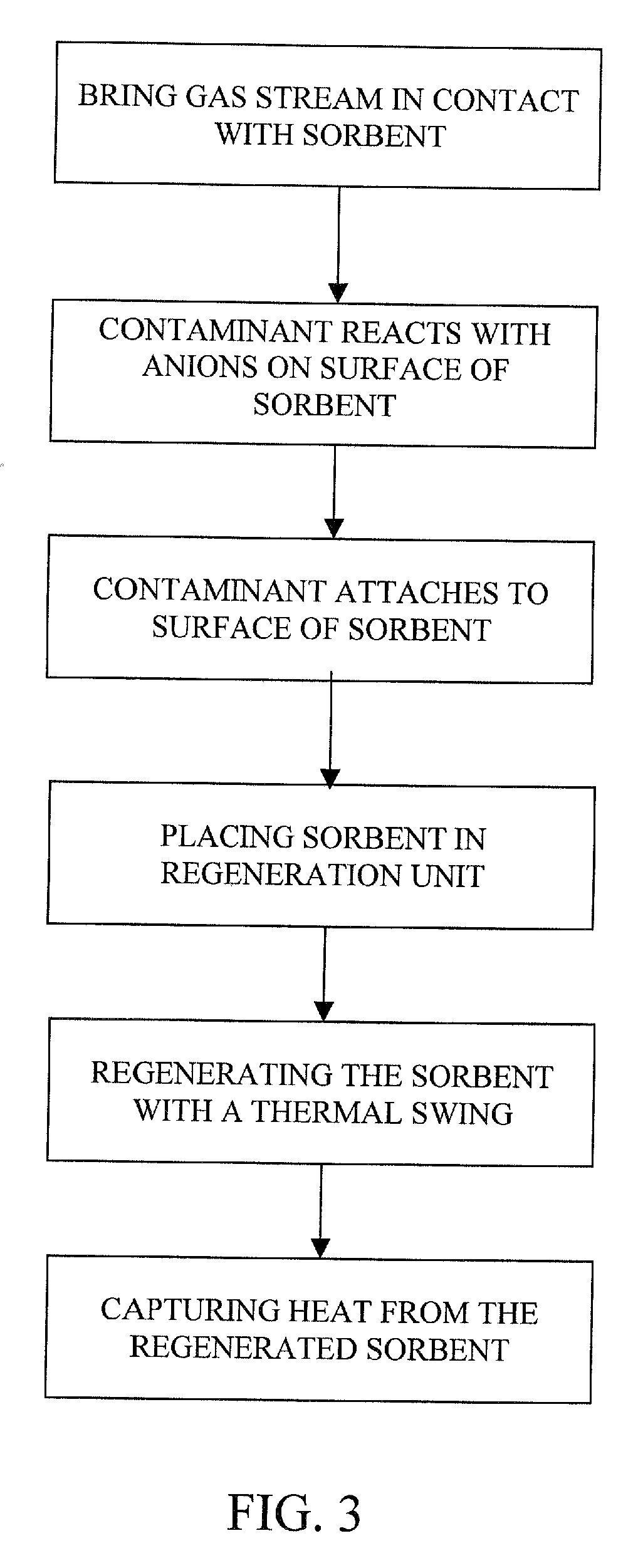
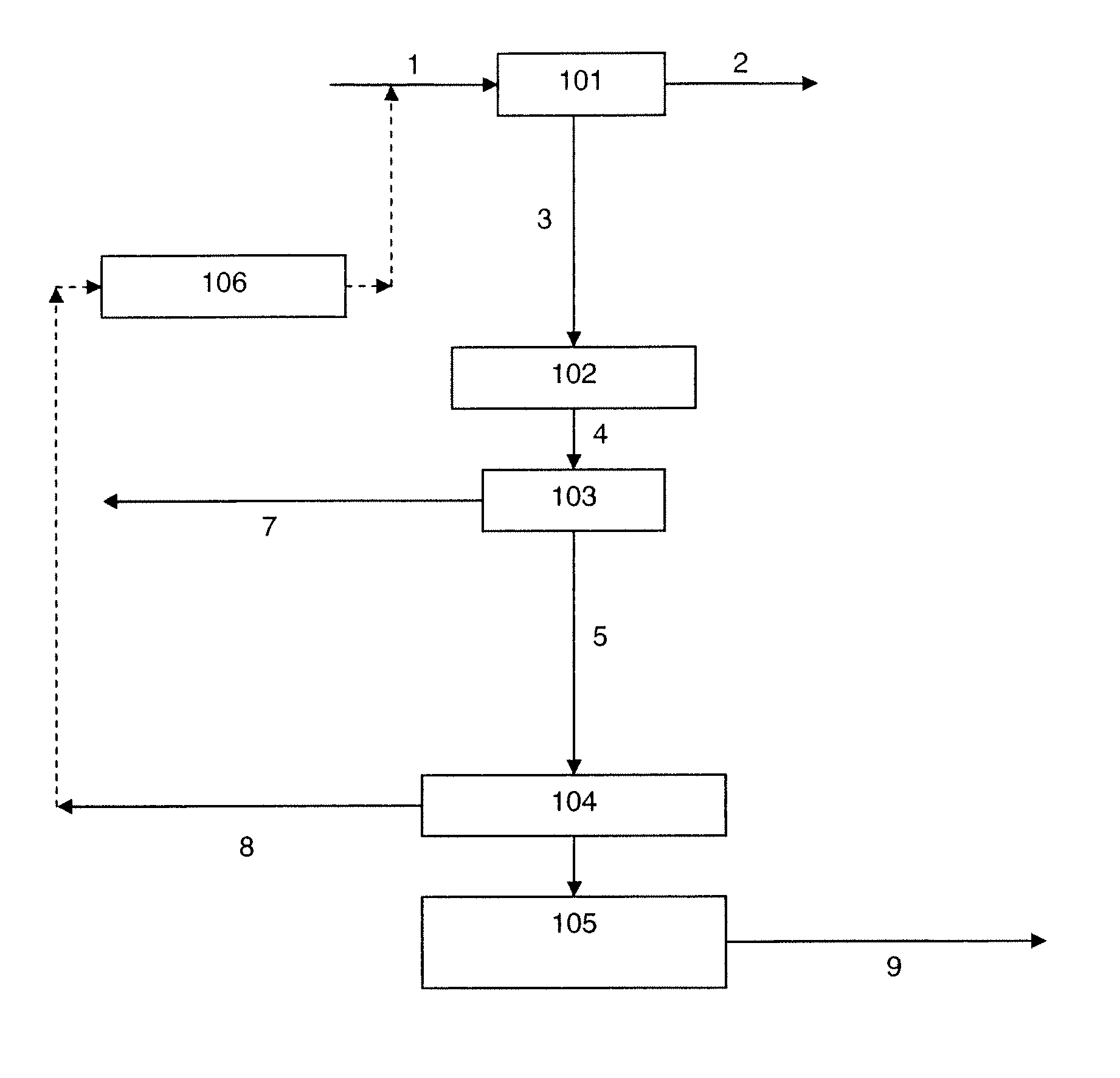
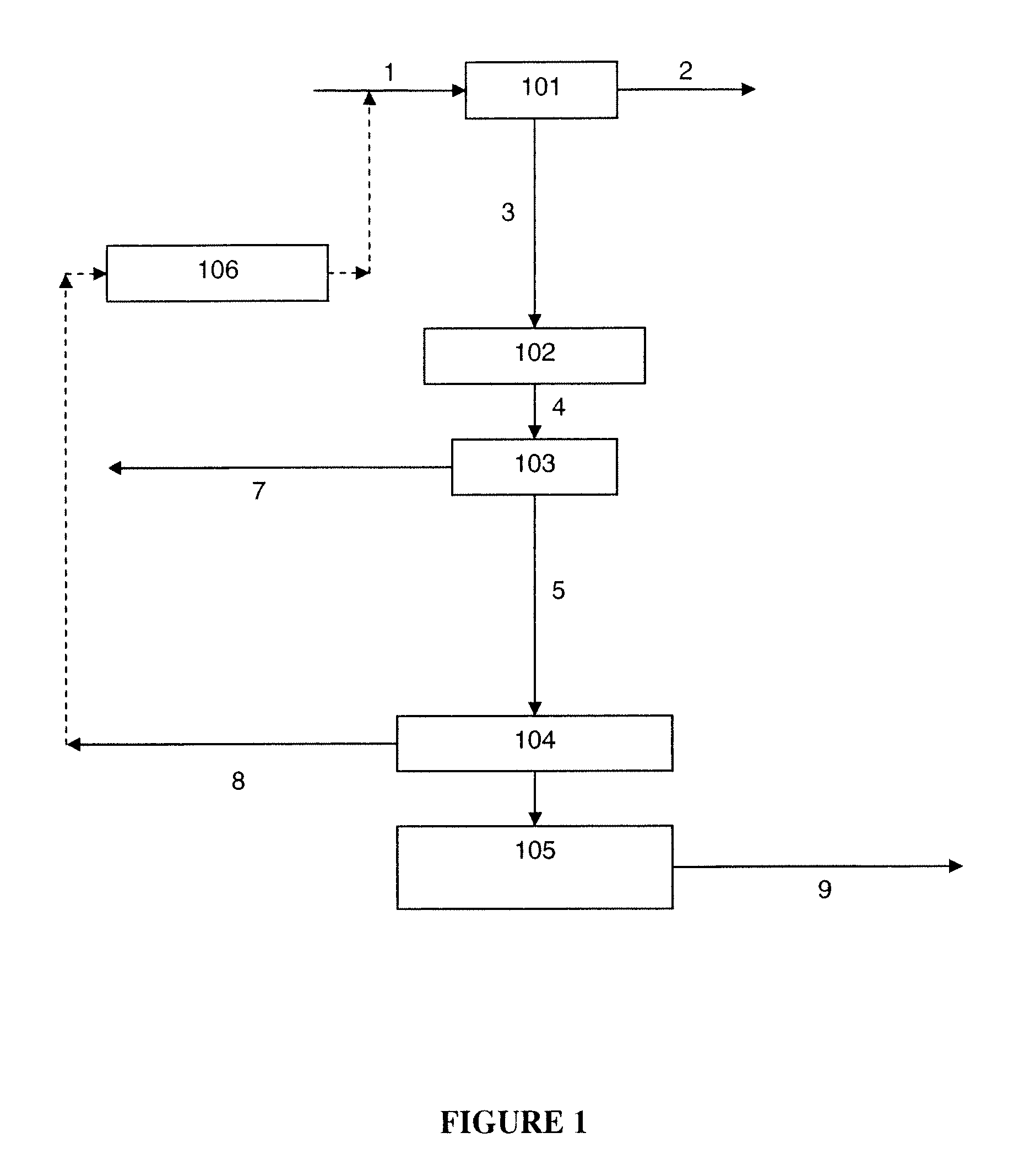
Removal of carbon dioxide from air
The present invention provides a method and apparatus for removing a contaminant, such as carbon dioxide, from a gas stream, such as ambient air. The contaminant is removed from the gas stream by a sorbent which may be regenerated using a humidity swing, a thermal swing, or a combination thereof. The sorbent may comprise a substrate having embedded positive ions and individually mobile negative ions wherein the positive ions are sufficiently spaced to prevent interactions between the negative ions. Where a thermal swing is used, heat may be conserved by employing a heat exchanger to transfer heat from the regenerated sorbent to an amount of sorbent that is loaded with the contaminant prior to regeneration.
Owner:CARBON SINK
Separation of a sour syngas stream
A feed stream, comprising hydrogen sulphide (H2S), carbon dioxide (CO2), hydrogen (H2) and, optionally, carbon monoxide (CO), is separated into at least a CO2 product stream and an H2 or H2 and CO product stream. The stream is separated using a pressure swing adsorption system, an H2S removal system and a further separation system, which systems are used in series to separate the stream. The method has particular application in the separation of a sour (i.e. sulphur containing) syngas, as for example produced from the gasification of solid or heavy liquid carbonaceous feedstock.
Owner:AIR PROD & CHEM INC
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Separation of Carbon Dioxide (Co2) From Gas Mixtures By Calcium Based Reaction Separation (Cars-Co2) Process
InactiveUS20080233029A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentSorbentTransformation ratio
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCOa). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Amine absorber for carbon dioxide capture and processes for making and using the same
InactiveUS20100263534A1Lower potentialIncreasing amineGas treatmentMolecular sieve catalystsSulfurAbsorbent material
Owner:THE UNIVERSITY OF AKRON
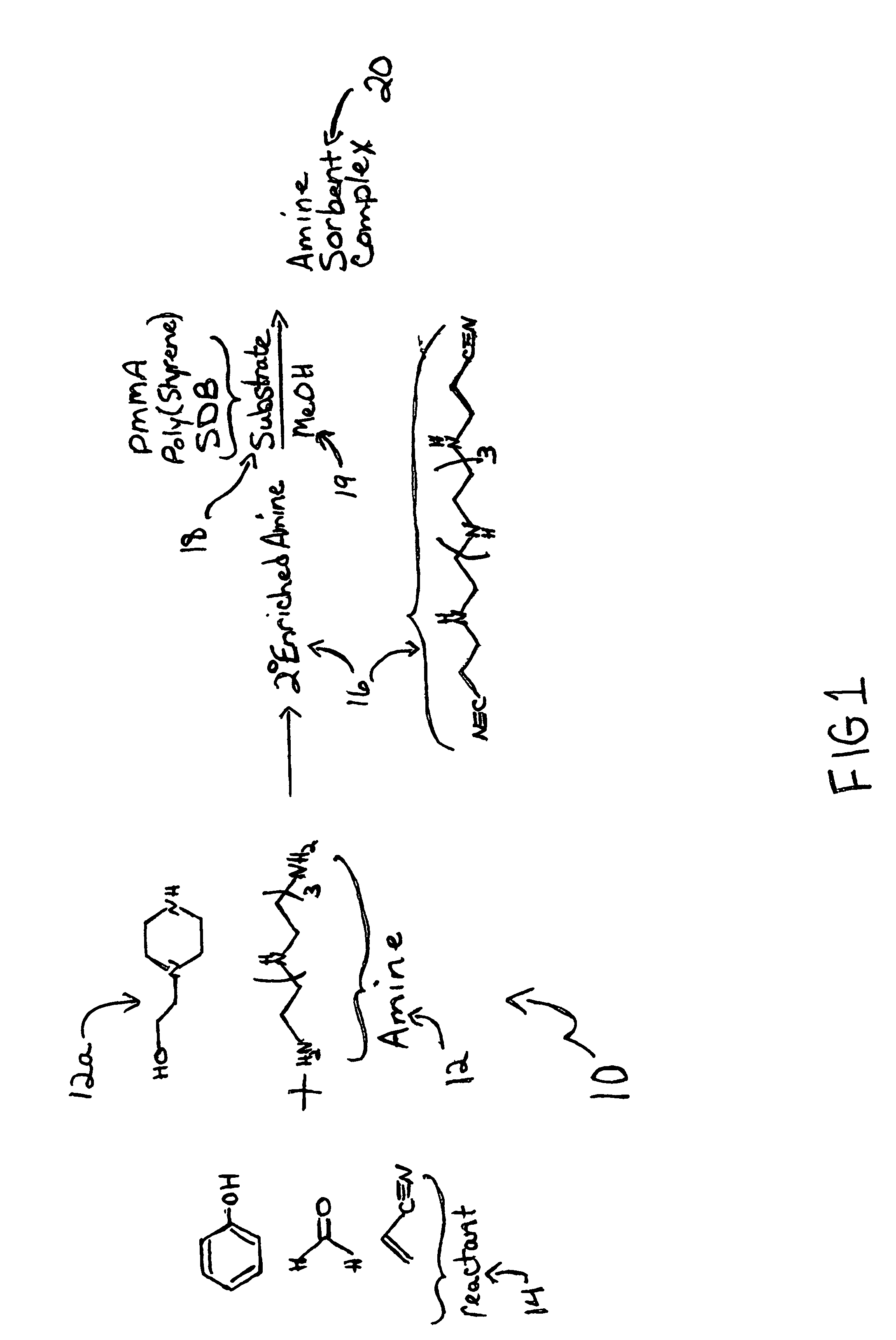
High capacity immobilized amine sorbents
ActiveUS7288136B1Increase capacityHigh CO capture capacityOther chemical processesHydrogen sulfidesSorbentImproved method
A method is provided for making low-cost CO2 sorbents that can be used in large-scale gas-solid processes. The improved method entails treating an amine to increase the number of secondary amine groups and impregnating the amine in a porous solid support. The method increases the CO2 capture capacity and decreases the cost of utilizing an amine-enriched solid sorbent in CO2 capture systems.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Gas separation by combined pressure swing and displacement purge
InactiveUS6902602B2Improve efficiencyAvoid pollutionGas treatmentIsotope separationSyngasAtmospheric air
Owner:AIR PROD & CHEM INC
Nano-structure supported solid regenerative polyamine and polyamine polyol absorbents for the separation of carbon dioxide from gas mixtures including the air
ActiveUS20080293976A1Increased CO absorption capacityIncreased COMaterial nanotechnologyElectrolysis componentsPolyolSorbent
The invention relates to regenerative, supported amine sorbents that includes an amine or an amine / polyol composition deposited on a nano-structured support such as nanosilica. The sorbent provides structural integrity, as well as high selectivity and increased capacity for efficiently capturing carbon dioxide from gas mixtures, including the air. The sorbent is regenerative, and can be used through multiple operations of absorption-desorption cycles.
Owner:UNIV OF SOUTHERN CALIFORNIA
Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room-temperature
InactiveUS20070068389A1Promote absorptionSufficiently flexibleInternal combustion piston enginesExhaust apparatusCo2 storageRoom temperature
A carbon dioxide storage system includes a container and a conduit attached to the container for introducing or removing a carbon dioxide-containing composition from the container. A carbon dioxide storage material is positioned within the container. The carbon dioxide-storage material includes a metal-organic framework, which has a sufficient surface area to store at least 10 carbon dioxide molecules per formula unit of the metal-organic framework at a temperature of about 25° C.
Owner:RGT UNIV OF MICHIGAN
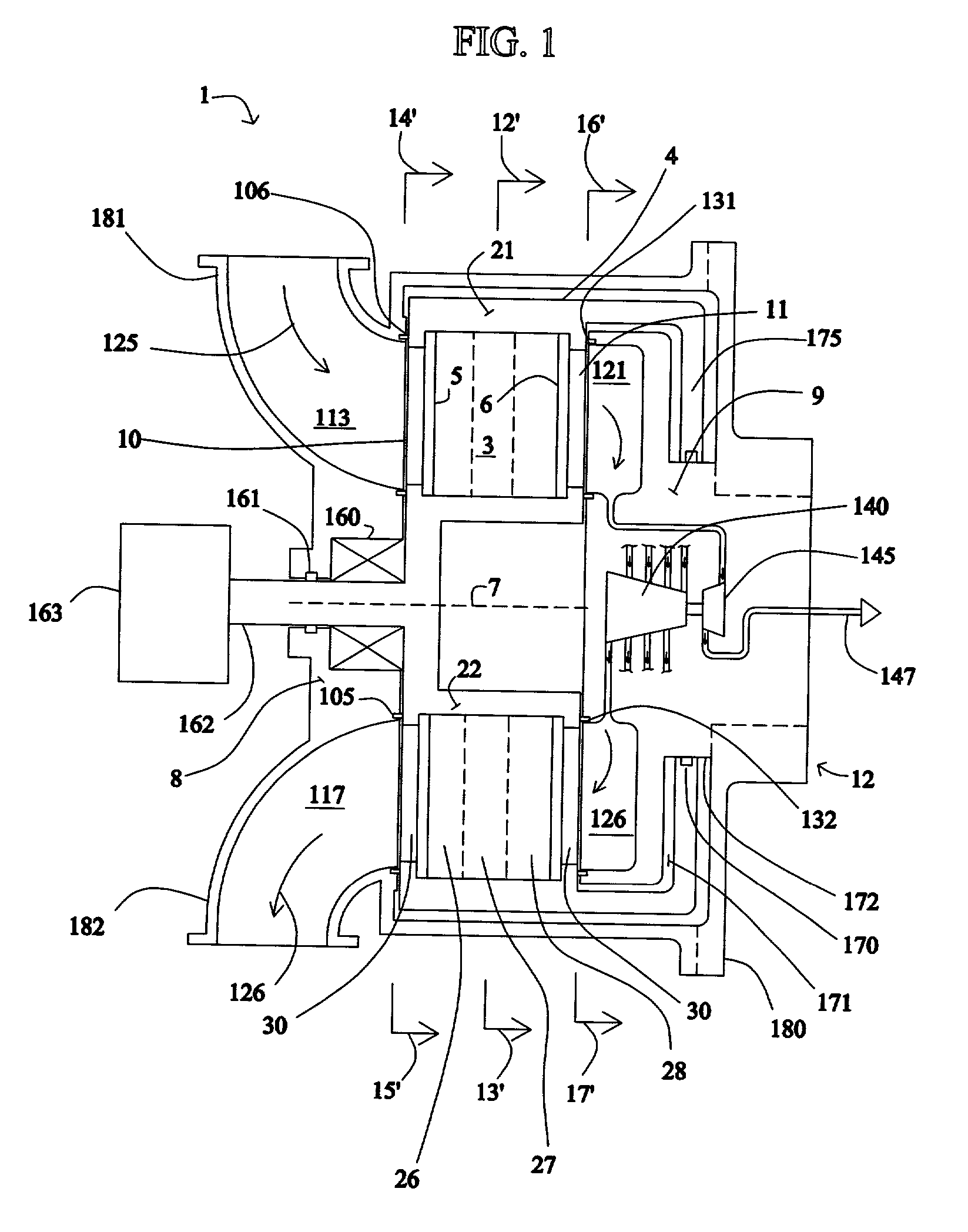
Electrical current generation system
InactiveUS6921597B2Facilitate pressureFacilitate ion exchangeFuel cell auxillariesHydrogen/synthetic gas productionHydrogenFuel cells
An electrical generating system consists of a fuel cell, and an oxygen gas delivery. The fuel cell includes and anode channel having an anode gas inlet for receiving a supply of hydrogen gas, a cathode channel having a cathode gas inlet and a cathode gas outlet, and an electrolyte in communication with the anode and cathode channel for facilitating ion exchange between the anode and cathode channel. The oxygen gas delivery system is coupled to the cathode gas inlet and delivers oxygen gas to the cathode channel. The electrical current generating system also includes gas recirculation means couple to the cathode gas outlet for recirculating a portion of cathode exhaust gas exhausted from the cathode gas outlet to the cathode gas inlet.
Owner:AIR PROD & CHEM INC
Carbon nanotube-containing structures, methods of making, and processes using same
InactiveUS7011760B2Large specific surface areaImprove conductivityCarbon compoundsChemical/physical/physico-chemical microreactorsPre treatmentSURFACTANT BLEND
Carbon nanotube structures are disclosed in which nanotubes are disposed over a porous support such as a foam, felt, mesh, or membrane. Techniques of making these structures are also disclosed. In some of these techniques, a support is pretreated with a templated surfactant composition to assist with the formation of a nanotube layer.
Owner:BATTELLE MEMORIAL INST
Systems and processes for providing hydrogen to fuel cells
InactiveUS20020098394A1Minimized in sizeMaximize energy efficiencyFuel cell auxillariesHydrogen/synthetic gas productionFuel cellsInternal combustion engine
A process and system for providing a hydrogen-containing gas stream to a fuel cell anode that includes providing a hydrogen-containing gas stream that includes carbon monoxide, introducing the hydrogen-containing gas stream into a pressure swing adsorption module that includes at least one carbon monoxide-selective adsorbent to produce a purified hydrogen-containing gas stream, and introducing the purified hydrogen-containing gas stream to the fuel cell anode. The pressure swing adsorption module can also include a second adsorbent and / or catalyst. Also disclosed is a fuel cell system coupled to an internal combustion engine and a fuel cell system that utilizes fuel cell waste heat for vaporizing a hydrocarbon / water mixture.
Owner:AIR PROD & CHEM INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com