1,2,3,4-Tetrahydronaphthalene compound and its preparation method and application
A compound, tetrahydronaphthalene technology, applied in the field of organic compound synthesis, can solve problems such as limited application range, harsh preparation conditions, and unfriendly environment, and achieve the effect of shortening the reaction route, requiring low reaction conditions, and reducing environmental pollution pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
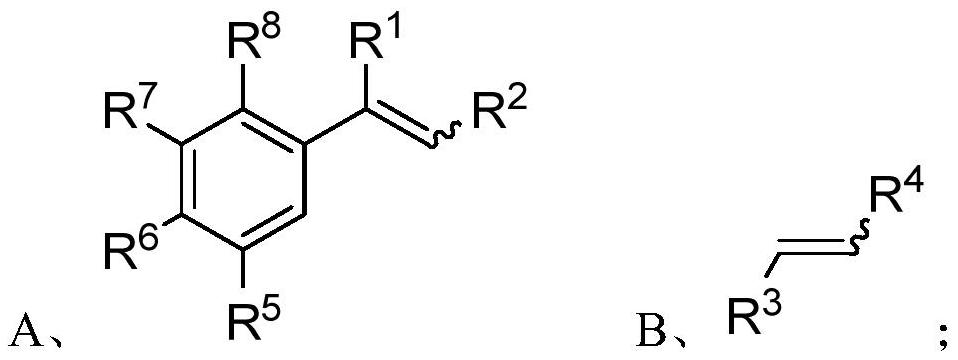
[0097] In one embodiment, the preparation method of the 1,2,3,4-tetrahydronaphthalene compound comprises the following steps:
[0098] S01: respectively provide the styrene compound A and the olefin compound B represented by the following structural formula:
[0099]
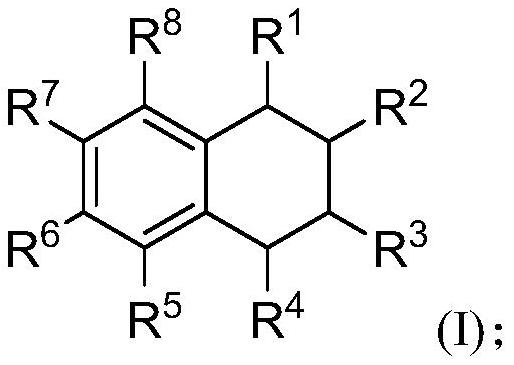
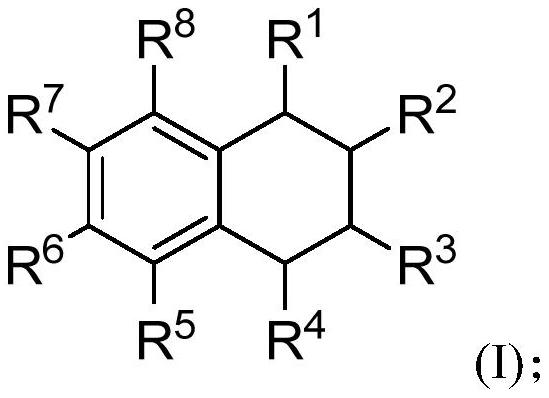
[0100] S02: Add the styrene compound A and the olefin compound B into a reaction system containing a visible light catalyst, a co-catalyst and a solvent to react at a temperature of -80-100°C to obtain the following structure represented by (I) The 1,2,3,4-tetrahydronaphthalene compounds shown,
[0101]
[0102]Specifically, in the above step S01, R in the molecular structural formula of styrene compound A 1 , R 2 , R 5 , R 6 , R 7 , R 8 The represented group is as above in the invention embodiment 1,2,3,4-tetrahydronaphthalene compound molecular structure general formula (I) in R 1 , R 2 , R 5 , R 6 , R 7 , R 8 The groups represented are the same.
[0103] R in the molecular structure formula...
Embodiment 1
[0136] Example 1 provides 1-(4-methoxyphenyl)-4-methyl-1,2,3,4-tetrahydronaphthalene and its preparation method.
[0137] The structural formula of the 1-(4-methoxyphenyl)-4-methyl-1,2,3,4-tetrahydronaphthalene is shown in the following molecular structural formula I1:
[0138]
[0139] Its preparation steps are as follows:
[0140] In a dry 10 mL test tube, add mesitylene-substituted acridinium salt visible light catalyst (0.01 mmol, 0.1 eq), diphenyl disulfide co-catalyst (0.01 mmol, 0.1 eq) and 2 mL of anhydrous dichloroethane, argon Replace three times, add 0.3mmola-methylstyrene, replace with argon three times again, seal the reaction test tube and stir at room temperature for 10 min. Dissolve p-methoxystyrene benzene (0.1mmol, 1.0eq) in 2mL of anhydrous dichloroethane, and slowly add it into the reaction system, while adding it, irradiate it with two blue LEDs with a power of 15w, add and react The time is 10h. After the reaction was completed, the filtrate was spi...
Embodiment 2
[0143] Example 2 provides 1-(4-methoxyphenyl)-4-ethyl-1,2,3,4-tetrahydronaphthalene and its preparation method. The structural formula of the 1-(4-methoxyphenyl)-4-ethyl-1,2,3,4-tetrahydronaphthalene is shown in molecular structural formula I2 below:
[0144]
[0145] Its preparation method refers to the preparation method of 1-(4-methoxyphenyl)-4-methyl-1,2,3,4-tetrahydronaphthalene in Example 1, the difference is that a-ethylstyrene (0.3mmol) instead of a-methylstyrene, the filtrate was spin-dried, and separated by column chromatography to obtain the target product as a colorless oily liquid with a yield of 77% and a dr value of 1:1.
[0146] The product I2 prepared is subjected to characterization data analysis, and the result is: 1 HNMR (300MHz, Chloroform-d) δ7.29(td, J=8.5, 1.5Hz, 1H), 7.19(tt, J=7.8, 2.1Hz, 1H), 7.12–6.98(m, 3H), 6.87(qd ,J=5.8,5.2,2.0Hz,3H), 4.09(t,J=11.1,7.0Hz,1H),3.82(s,3H),2.83(ddq,J=19.4,9.8,5.3Hz,1H), 2.24(dddd,J=12.6,8.7,5.6,2.8Hz,1H),2.10–...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com