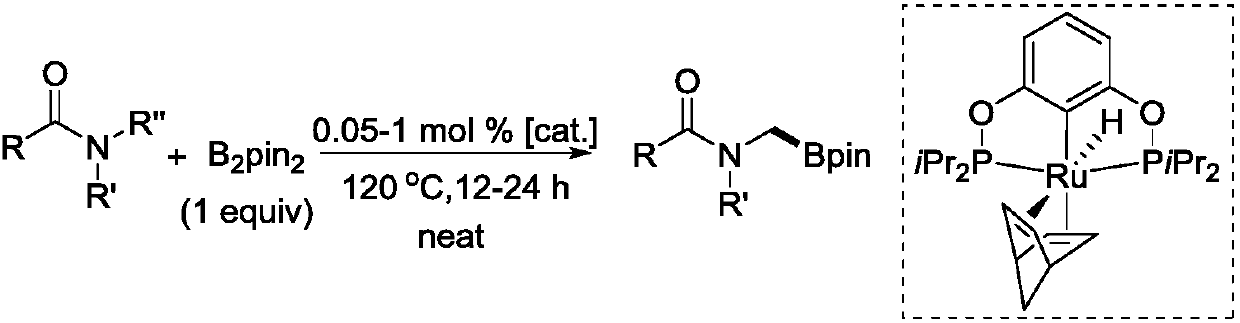
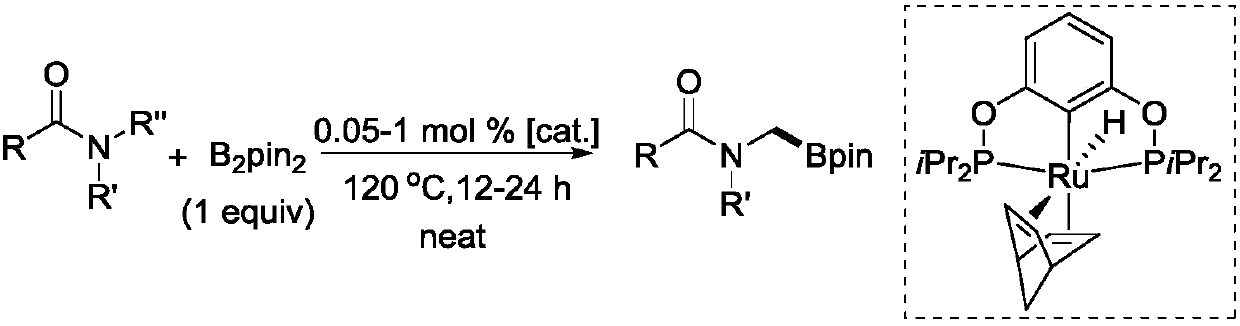
Novel method for ruthenium-catalyzed selective boronation reaction of amides
A ruthenium-catalyzed amide and selective technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as limited application value, poor atom economy, and limited reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
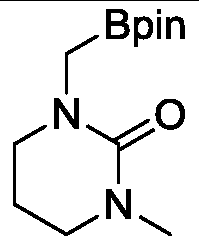
[0019] Example 1, wherein the N,N-disubstituted amide substrate is as follows:
[0020] Amide substrate structure formula:
[0021]
[0022] In an argon glove box, add metal ruthenium complex (3.0 mg, 5.6 μmol), amide substrate (11.2 mmol), B 2 pin 2 (2.8 g, 11.2 mmol). Next, the 10 mL sealed tube was tightened and removed from the glove box, and placed in an oil bath at 120° C. for heating and stirring for 24 hours. When the reaction solution was cooled to room temperature, the reaction was quenched with ethyl acetate, and then the low-boiling point organic matter was sucked dry on a rotary evaporator. Finally, methylene bromide in an equimolar amount to the substrate was added as an internal standard to measure the NMR yield of the product, and after obtaining the NMR yield, the low-boiling organic matter in the crude product was extracted again. Purification by column separation, the eluent used is ethyl acetate:methanol=1:0-5:1. product of 1 H NMR yield: 98%, isol...
Embodiment 2
[0023] Example 2, wherein the N,N-disubstituted amide substrate is as follows:
[0024] Amide substrate structure formula:
[0025]
[0026] In an argon glove box, add metal ruthenium complex (3.0 mg, 5.6 μmol), amide substrate (11.2 mmol), B 2 pin 2 (2.8 g, 11.2 mmol). Next, the 10 mL sealed tube was tightened and removed from the glove box, and placed in an oil bath at 120° C. for heating and stirring for 24 hours. When the reaction solution was cooled to room temperature, the reaction was quenched with ethyl acetate, and then the low-boiling point organic matter was sucked dry on a rotary evaporator. Finally, methylene bromide in an equimolar amount to the substrate was added as an internal standard to measure the NMR yield of the product, and after obtaining the NMR yield, the low-boiling organic matter in the crude product was extracted again. White solid, the eluent used for passing through the column is ethyl acetate:petroleum ether=1:1-1:0. product of 1 H NMR ...
Embodiment 3
[0027] Example 3, wherein the N,N-disubstituted amide substrate is as follows:
[0028] Amide substrate structure formula:
[0029]
[0030] In an argon glove box, add metal ruthenium complex (3.0 mg, 5.6 μmol), amide substrate (11.2 mmol), B 2 pin 2 (2.8 g, 11.2 mmol). Next, the 10 mL sealed tube was tightened and removed from the glove box, and placed in an oil bath at 120° C. for heating and stirring for 24 hours. When the reaction solution was cooled to room temperature, the reaction was quenched with ethyl acetate, and then the low-boiling point organic matter was sucked dry on a rotary evaporator. Finally, methylene bromide in an equimolar amount to the substrate was added as an internal standard to measure the NMR yield of the product, and after obtaining the NMR yield, the low-boiling organic matter in the crude product was extracted again. White oil, the eluent used for passing through the column is ethyl acetate:petroleum ether=1:1-1:0. product of 1 H NMR yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com