Dendritic hyperbranched polymer as well as preparation method and use thereof
A kind of hyperbranched polymer and dendritic technology, which is applied in the direction of instruments, optics, nonlinear optics, etc., can solve the problems that are difficult to meet the requirements of future communication technology, transmission speed, capacity and space compatibility limit, and achieve good Three-dimensional bit separation effect, high second-order nonlinear optical effect, stability, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
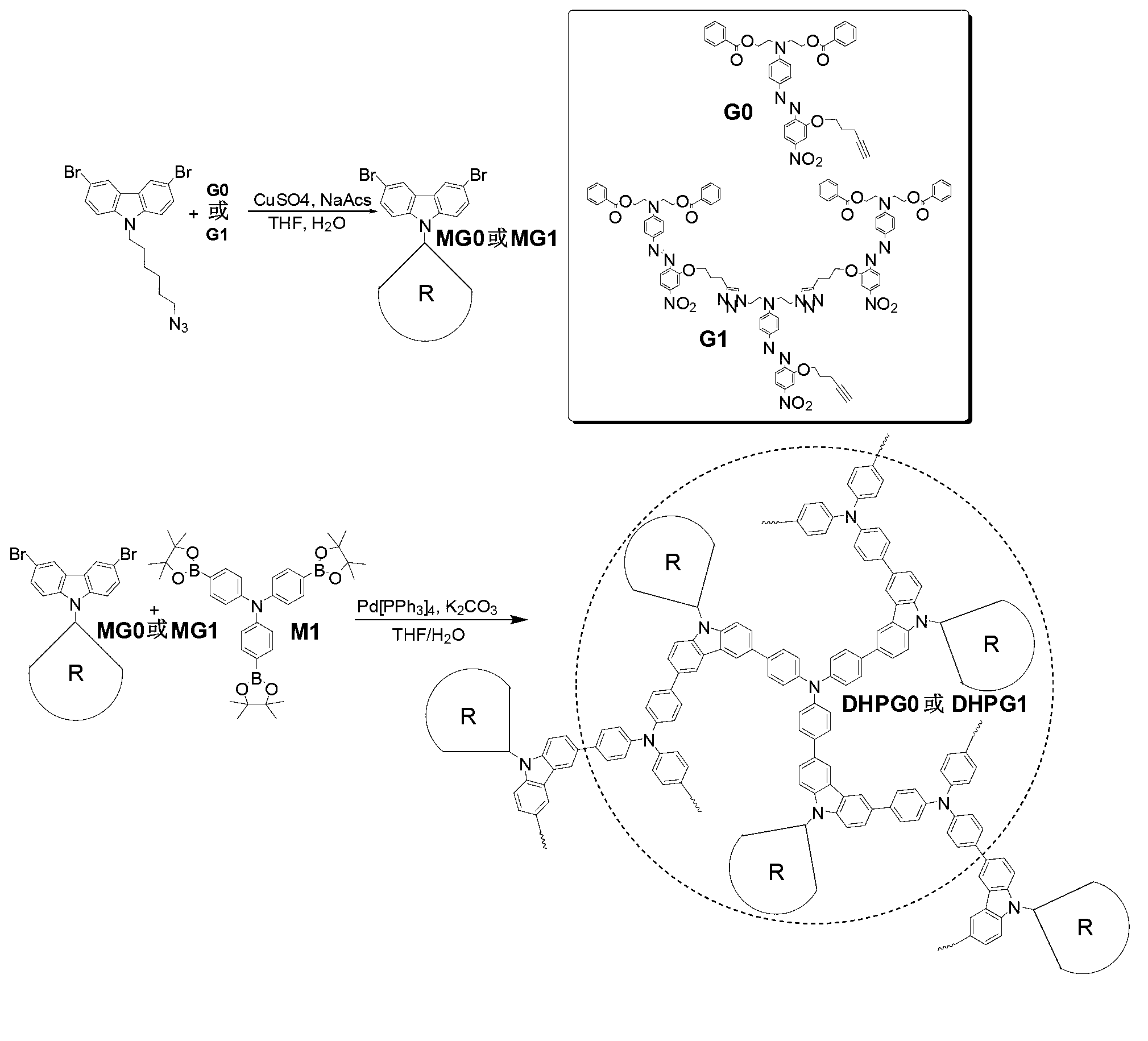
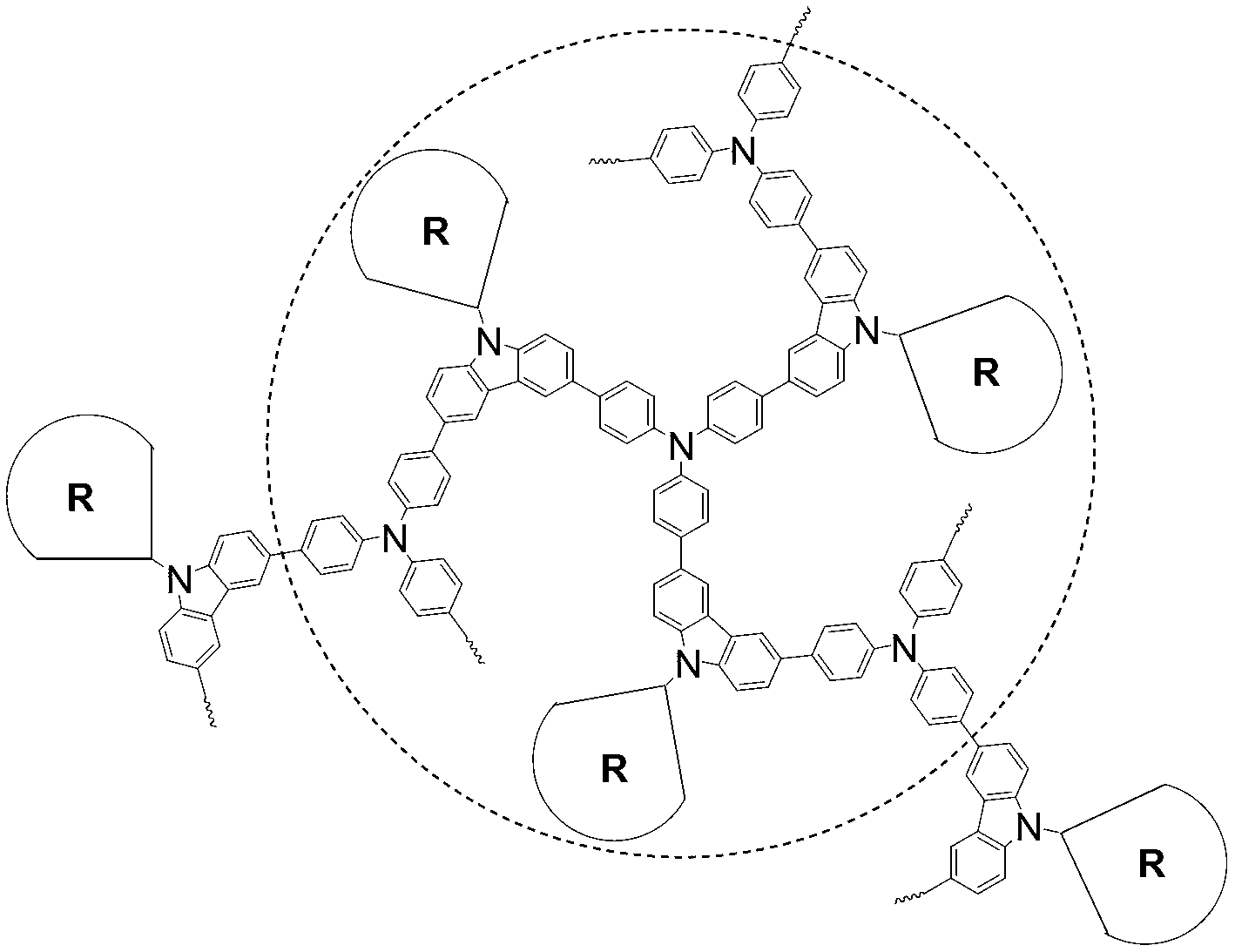
[0032] Synthesis of dendritic hyperbranched polymer DHPG0:
[0033] 1) In a 50mL Schlenk tube, add compound G0 (124.1mg, 2mmol), N-(6-azido)-3,6-dibromocarbazole (99mg, 2.2mmol), CuSO 4 ·5H 2 O (10mol%), 20% NaHCO 3 (20mol%) and sodium ascorbate (20mol%). Under argon protection, 5 mL of THF and 1 mL of water were added after deoxygenation, and reacted at room temperature for 3 hours. Water was added to the reaction system to quench the reaction. Extract with chloroform, combine the organic phases and spin dry with a rotary evaporator. The crude product was separated by silica gel column chromatography using chloroform / ethyl acetate (2 / 1, V / V) as the eluent to obtain 102 mg of a red solid with a yield of 95%.
[0034] IR(KBr),υ(cm -1 ):1723(C=O),1511,1341(-NO 2 ).
[0035] 1 H NMR (CDCl 3 ,300MHz)δ(TMS,ppm):1.26(s,br,4H,-CH 2 -),1.78(m,4H,-CH 2 -),2.32(t,J=6.9Hz,2H,-CH 2 C-),2.99(t,J=7.2Hz,2H,-CH 2 -),3.94(t,J=5.4Hz,2H,-NCH 2 -),4.21(t,J=6Hz,4H,-NCH 2 -),4.26(t,...
Embodiment 2
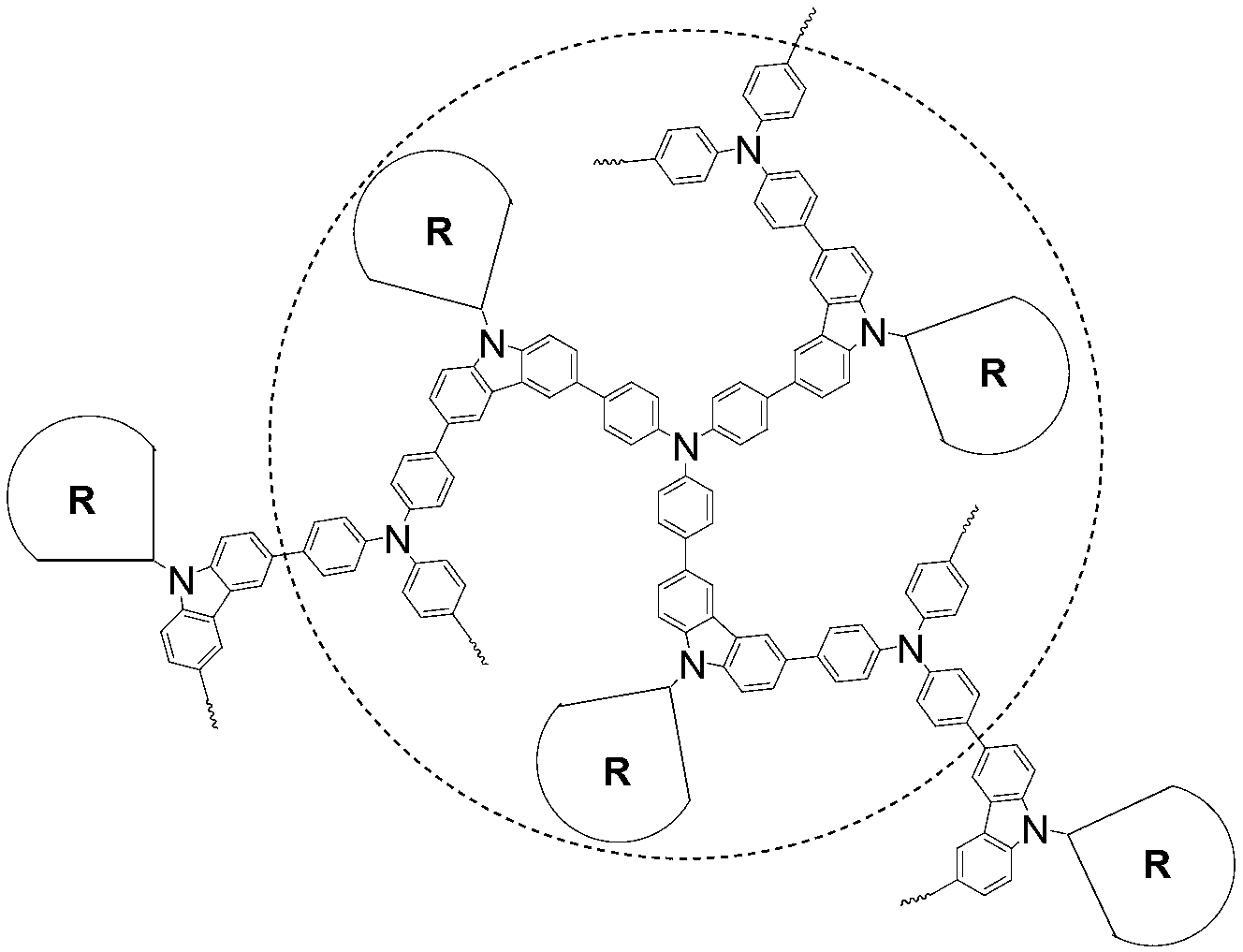
[0044] Synthesis of dendritic hyperbranched polymer DHPG1:
[0045] 1) In a 50mL Schlenk tube, add compound G1 (170.9mg, 1mmol), N-(6-azidohexyl)-3,6-dibromocarbazole (90mg, 2mmol), and add 5mL THF after deoxygenation, 0.25mL of 0.04mmol / mL CuSO 4 (10mol%) aqueous solution, 0.25mL of 0.08mmol / mL sodium pitascorbate (20mol%) aqueous solution, reacted at room temperature for 3 hours. An appropriate amount of water was added to the reaction system to quench the reaction. Extract with chloroform, combine the organic phases and spin dry with a rotary evaporator. The crude product was separated by silica gel column chromatography using chloroform / ethyl acetate (2 / 1, V / V) as the eluent to obtain 200 mg of a red solid with a yield of 93%.
[0046] IR(KBr),υ(cm -1 ):1723(C=O),1511,1341(-NO 2 ).
[0047] 1 H NMR (CDCl 3 ,300MHz)δ(TMS,ppm):0.94(t,J=7.2Hz,4H,-CH 2 -),1.26(s,br,4H,-CH 2 -),1.77(s,br,2H,-CH 2 C-),1.86(s,br,2H,-CH 2 -),2.22-2.30(m,4H,-CH 2 -),2.26(t,J=6.9Hz,4H,-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com