Lipopolysaccharide amine cationic polymer and preparation method and application thereof
A cationic polymer and lipopolysaccharide technology, applied in the field of biomedicine, can solve the problems of low gene transfection efficiency, virus transfection level, low transfection efficiency, etc., and achieve excellent biocompatibility, reduce cytotoxicity, and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
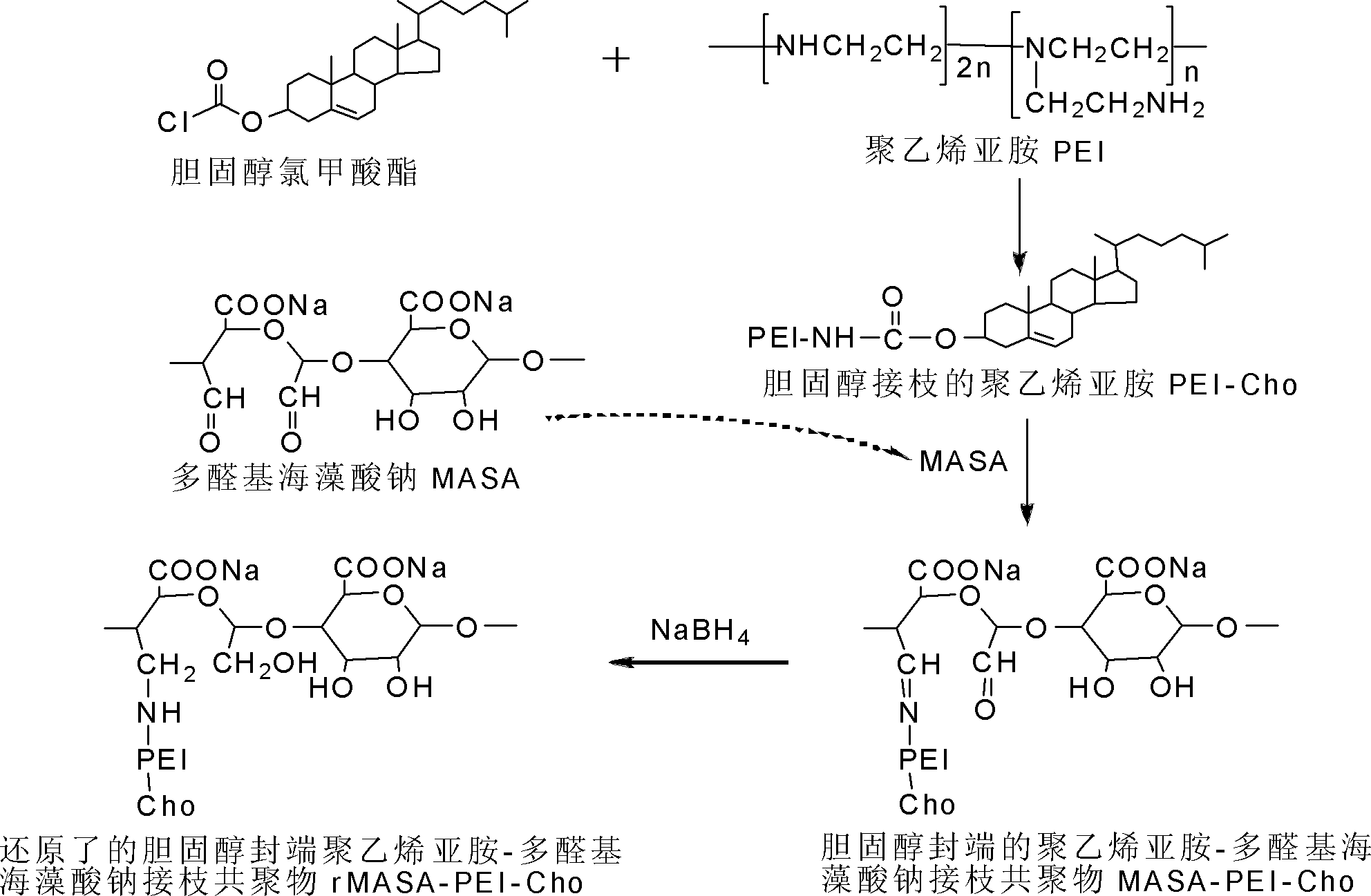
[0043] 1 Synthesis of reduced lipopolysaccharide amine cationic polymer rMASA-PEI-Cho
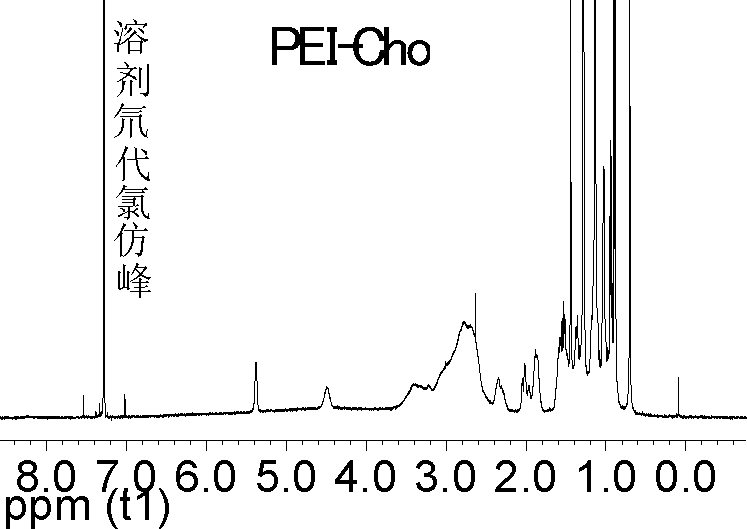
[0044] 1.1 Synthesis of cholesterol-terminated polyethyleneimine (PEI-Cho)
[0045] First, the methylene chloride and PEI with a molecular weight of less than 2k are dehydrated, and then 3g PEI (1.67×10 -3 mol), 10mL dichloromethane, 100μL triethylamine (7.17×10 -3 mol) fully stirred and mixed on ice for 30min to obtain a clear and transparent solution A; take 0.75g cholesterol chloroformate (1.67×10 -3 mol, where the molar feed ratio of PEI to cholesterol chloroformate is 1:1. ) Dissolve in 5 mL of ice-cold anhydrous dichloromethane to obtain a clear and clear solution B. Under stirring, solution 2 was slowly added dropwise to solution A within 30 minutes, and then the reaction mixture was allowed to continue stirring and reacting for 12 hours on ice, and the solvent was removed by rotary evaporation to obtain a white extremely viscous semi-solid. The semi-solid was dissolved in 50 mL of 0.1 m...
Embodiment 2
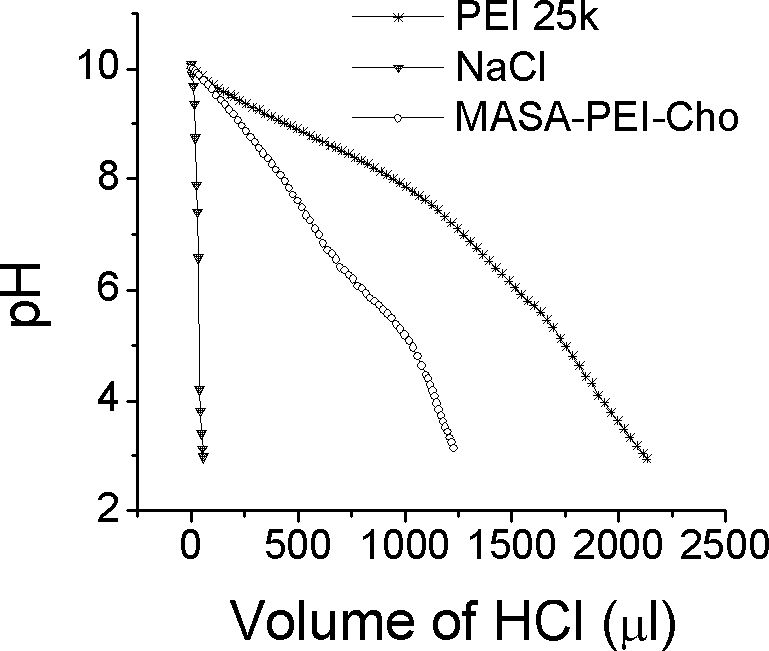
[0054] 1 Synthesis of lipopolysaccharide amine cationic graft copolymer MASA-PEI-Cho
[0055] 1.1 Synthesis of cholesterol-terminated polyethyleneimine (PEI-Cho)
[0056] First, the methylene chloride and PEI are dehydrated, and then 3g PEI (1.67×10 -3 mol), 10mL dichloromethane, 200μL triethylamine (1.43×10 -2 mol) Stir and mix well on ice for 30 minutes to obtain a clear and transparent solution A; take 1.5 g of cholesterol chloroformate (its molar ratio to PEI is 2:1) and dissolve it in 10 mL of ice-cold anhydrous dichloromethane to obtain a clear and clear solution B. Under stirring, solution B was slowly added dropwise to solution A within 30 minutes, and then the reaction mixture was allowed to continue stirring and reacting for 12 hours on ice, and the solvent was removed by rotary evaporation to obtain a white extremely viscous semi-solid. The semi-solid was dissolved in 50 mL 0.5 mol / L HCl, filtered, and the filtrate was extracted 3 times with 100 mL dichloromethane to re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com