Schiff base rare earth ytterbium iodide, and preparation method and application thereof
A technology of iodide and Schiff base is applied in the field of metal organic compound preparation to achieve the effects of low toxicity, easy purification and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
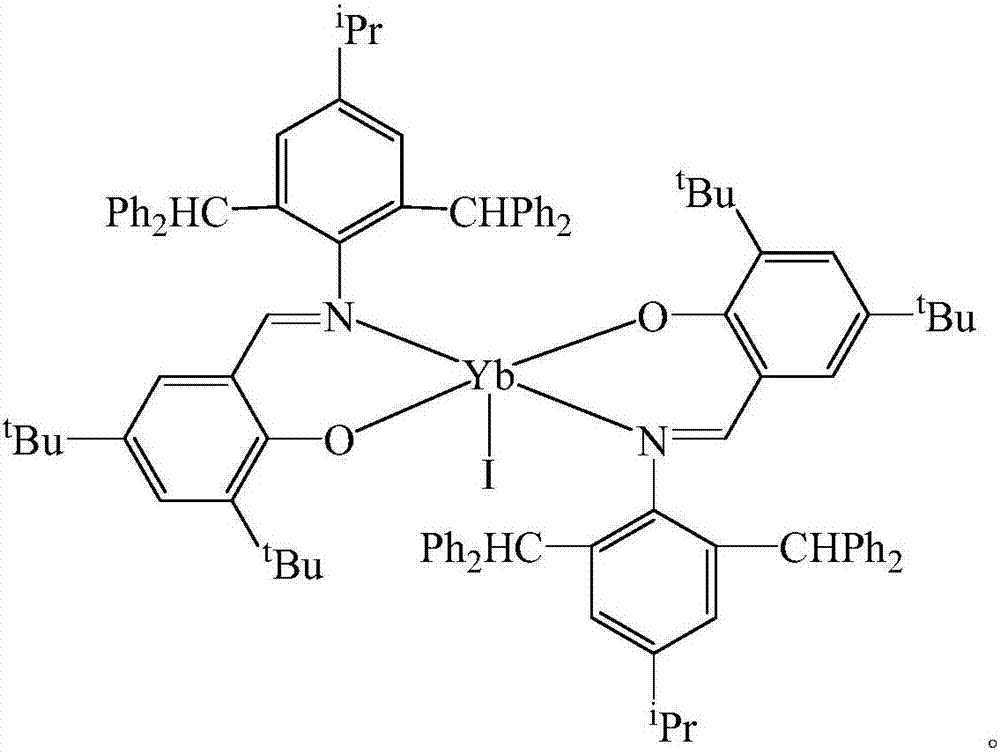
[0022] The preparation of N-(4-isopropyl-2,6-di(benzhydryl)phenyl)-3,5-di-tert-butyl-2-oxo-Schiff base rare earth ytterbium iodide is as follows:
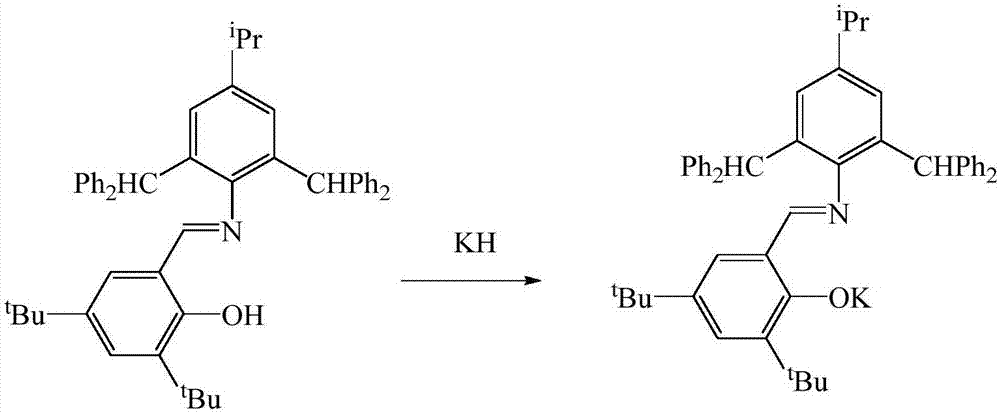
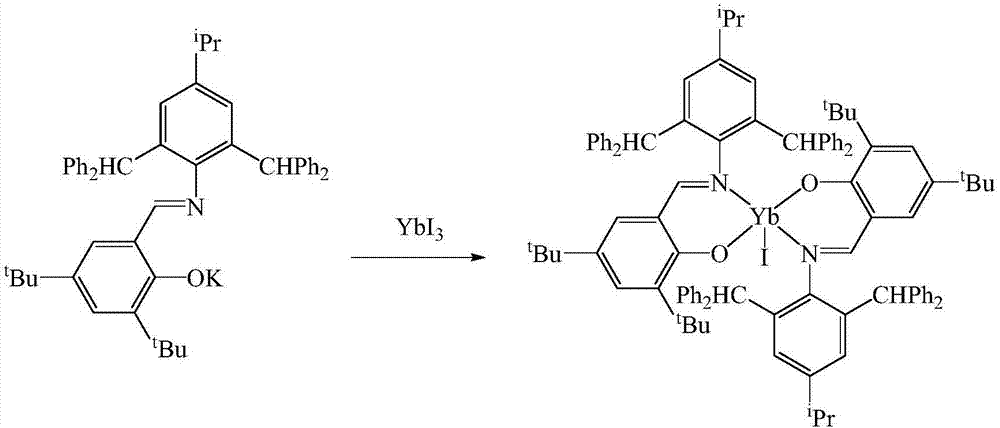
[0023] In the absence of water and oxygen, in a single-port reaction tube, N-(4-isopropyl-2,6-bis(benzhydryl)phenyl)-3,5-di-tert-butyl-2-hydroxybenzaldehyde Dissolve imine 1.46mmol in 40mL tetrahydrofuran solution at -80°C, dissolve potassium hydride 1.46mmol in 5mL tetrahydrofuran and add dropwise, react at room temperature for 2h, dissolve ytterbium triiodide 1.46mmol in 20mL tetrahydrofuran, add dropwise at -80°C The above solution was reacted for 10 h, the solvent was drained, added to 40 mL of toluene for extraction, and concentrated to 10 mL. After a few days, yellow crystals were obtained. After filtration, the mass was 0.82 g, and the yield was 60%. M.p. 282-284°C. Elemental Analysis: C 100 h 104 IN 2 o 2 Yb: C, 72.10; H, 6.29; N, 1.68. Found C, 72.58; H, 6.63; N, 1.32. Infrared: IR(KBr,cm -1 ):3429,3061,3028,2960,28...
Embodiment 2
[0025] The preparation of N-(4-isopropyl-2,6-di(benzhydryl)phenyl)-3,5-di-tert-butyl-2-oxo-Schiff base rare earth ytterbium iodide is as follows:
[0026] In the absence of water and oxygen, in a single-port reaction tube, N-(4-isopropyl-2,6-bis(benzhydryl)phenyl)-3,5-di-tert-butyl-2-hydroxybenzaldehyde Dissolve imine 1.75mmol in 40mL tetrahydrofuran solution at -60°C, dissolve potassium hydride 2.10mmol in 5mL tetrahydrofuran and add dropwise, react at room temperature for 3h, dissolve ytterbium triiodide 1.75mmol in 20mL tetrahydrofuran, add dropwise at -60°C The above solution was reacted for 15 hours, the solvent was drained, added to 40 mL of toluene for extraction, and concentrated to 10 mL. After a few days, yellow crystals were obtained. After filtration, the mass was 0.98 g, and the yield was 59%. M.p. 282-284°C. Elemental Analysis: C 100 h 104 IN 2 o 2 Yb: C, 72.10; H, 6.29; N, 1.68. Found C, 72.58; H, 6.63; N, 1.32. Infrared: IR (KBr, cm -1):3429,3061,3028,296...
Embodiment 3
[0028] The preparation of N-(4-isopropyl-2,6-di(benzhydryl)phenyl)-3,5-di-tert-butyl-2-oxo-Schiff base rare earth ytterbium iodide is as follows:
[0029] In the absence of water and oxygen, in a single-port reaction tube, N-(4-isopropyl-2,6-bis(benzhydryl)phenyl)-3,5-di-tert-butyl-2-hydroxybenzaldehyde Dissolve imine 1.75mmol in 40mL tetrahydrofuran solution at -40°C, dissolve potassium hydride 2.10mmol in 5mL tetrahydrofuran and add dropwise, react at room temperature for 4h, dissolve ytterbium triiodide 1.75mmol in 20mL tetrahydrofuran, add dropwise at -40°C The above solution was reacted for 20 hours, the solvent was drained, added to 40 mL of toluene for extraction, and concentrated to 10 mL. After a few days, yellow crystals were obtained, filtered, and the mass was 0.99 g, with a yield of 61%. M.p. 282-284°C. Elemental Analysis: C 100 h 104 IN 2 o 2 Yb: C, 72.10; H, 6.29; N, 1.68. Found C, 72.58; H, 6.63; N, 1.32. Infrared: IR (KBr, cm -1 ):3429,3061,3028,2960,286...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com