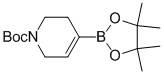
Method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester
A technology of tert-butoxycarbonyl and tetrahydropyridine, which is applied in the field of chemical synthesis, can solve the problems of low yield, long cycle and high cost, and achieve the effects of short process route, high yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
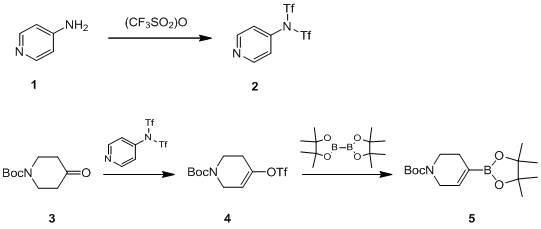
[0038] (1) Synthesis of nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide
[0039] Dissolve 4-aminopyridine and triethylamine in a molar ratio of 1:3 in dried methylene chloride (the amount used is 10 times the mass of 4-aminopyridine), and slowly add the molar amount of 4-aminopyridine dropwise at 0°C 2.2 times of trifluoromethanesulfonic anhydride, dropwise for 1 hour, stir at room temperature for 1 hour after dropping, cool to 0°C, wash the solution with water and saturated brine, dry the dichloromethane layer with anhydrous sodium sulfate, and evaporate to dryness , in crude nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide. The crude product was recrystallized from n-hexane to obtain the pure product with a yield of 80%.
[0040] (2) Synthesis of tert-butyl 3,6-dihydro-4-[[(trifluoromethyl)sulfonyl]oxy]-1(2H)-pyridinecarboxylate
[0041] Dissolve diisopropylamine in dry tetrahydrofuran (the amount of tetrahydrofuran is 10 times the mass of diisopropylamine), a...
Embodiment 2
[0048] (1) Synthesis of nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide
[0049] Dissolve 4-aminopyridine and triethylamine in a molar ratio of 1:3.5 in dried methylene chloride (the amount used is 10 times the mass of 4-aminopyridine), and slowly add the molar amount of 4-aminopyridine dropwise at 0°C 2.5 times the amount of trifluoromethanesulfonic anhydride, the dropwise addition time is 2 hours, after the dropping is completed, stir at room temperature for 1 hour; cool to about 0°C, wash the solution with water and saturated saline, dry the dichloromethane layer with anhydrous sodium sulfate, evaporate Dry to get the crude product nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide, the crude product nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide was recrystallized with n-hexane to obtain pure product, yield 85% .
[0050] (2) Synthesis of tert-butyl 3,6-dihydro-4-[[(trifluoromethyl)sulfonyl]oxy]-1(2H)-pyridinecarboxylate
[0051] Dissolve diisopropylam...
Embodiment 3
[0058] (1) Synthesis of nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide
[0059] Dissolve 4-aminopyridine and triethylamine in dry dichloromethane at a molar ratio of 1:4 (the amount of dichloromethane is 13 times the mass of 4-aminopyridine), slowly add 4 -Trifluoromethanesulfonic anhydride with 2.2 times the molar weight of aminopyridine, the dropwise addition time is 1 hour; after the dropping is completed, stir at room temperature for 1 hour, cool to 5°C, wash the solution with water and saturated brine, and wash the dichloromethane layer with anhydrous sulfuric acid Drying over sodium and evaporating to dryness gave the crude product nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide, and the crude product nitrogen-4-pyridine bis(trifluoromethanesulfonyl)imide was recrystallized with n-hexane to obtain the pure product, The yield is 81%.
[0060] (2) Synthesis of tert-butyl 3,6-dihydro-4-[[(trifluoromethyl)sulfonyl]oxy]-1(2H)-pyridinecarboxylate
[0061] Diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com