Mazarine electroluminescent compound, and preparation method and application thereof
An electroluminescence and compound technology, which is applied in the field of organic electroluminescence and can solve the problems of high device startup voltage and rare organic light-emitting materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
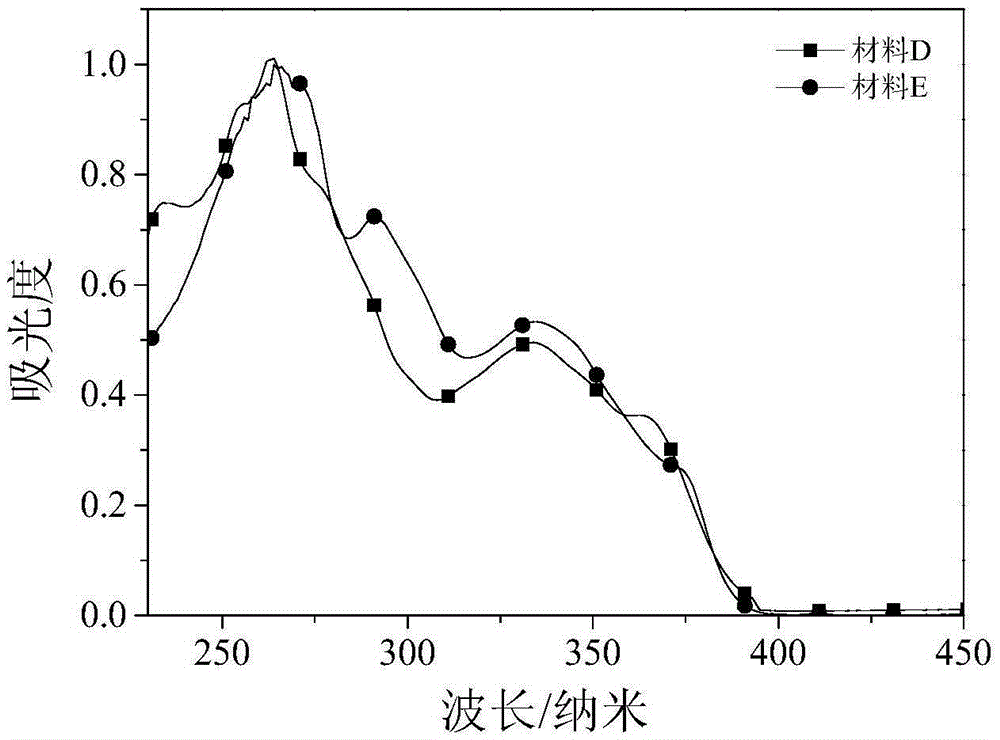
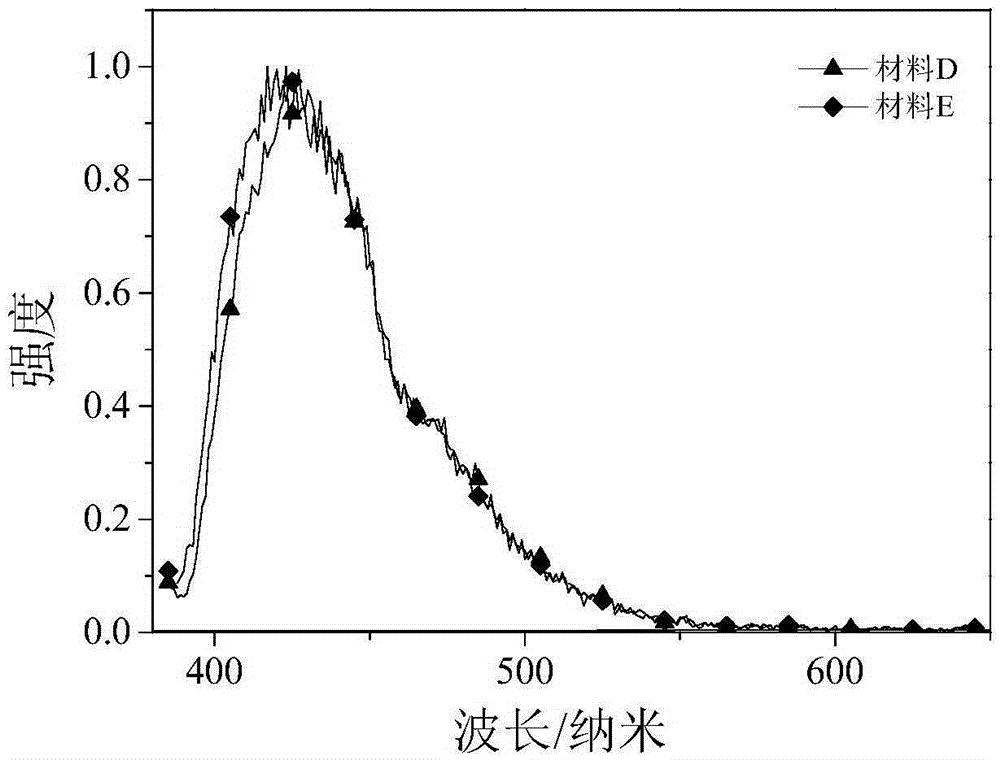
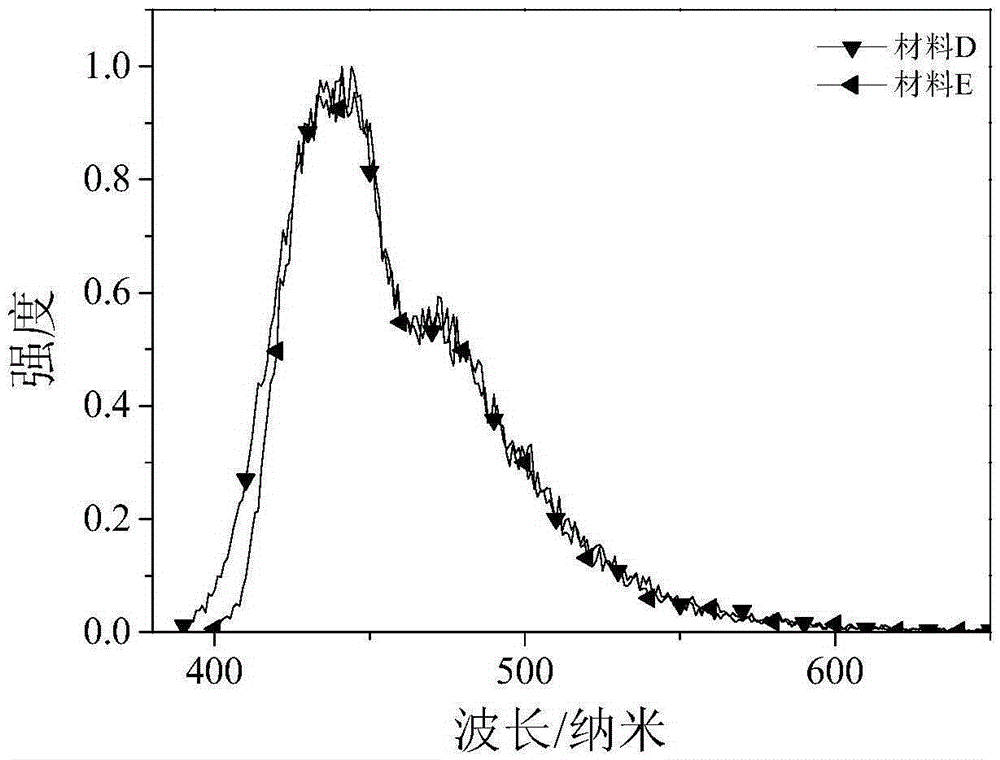
[0035] The dark blue organic luminescent material prepared in this example is: 2-(4-dibenzofuran-4-phenyl)-1-(4-methoxyphenyl)phenanthroimidazole, whose molecular formula is C 40 h 26 N 2 o 2 , the structural formula is:
[0036]
[0037] Concrete preparation steps are as follows:
[0038]
[0039] 1. Preparation of precursor dibenzofuran-4-pinacol borate
[0040]Under the protection of argon, dissolve 10-12g of dibenzofuran in 100-200mL of tetrahydrofuran solvent, and stir evenly at a speed of 650r / min to obtain a mixed solution; put the reaction system in a dry ice acetone bath to keep low temperature, Add 30-45 mL of n-butyllithium n-hexane solution dropwise to the above mixed solution at a rate of 10 drops / min to 20 drops / min. As for adding 25–30 g of 1,2-dibromoethane to the tetrahydrofuran solvent in the dry ice acetone bath, after the dropwise addition, the system naturally returns to room temperature and stirs for 10–15 hours, then removes the solvent with c...
Embodiment 2
[0046] The dark blue organic luminescent material prepared in this example is: 2-(4-dibenzothiophene-4-phenyl)-1-(4-methoxyphenyl)phenanthroimidazole, whose molecular formula is C 40 h 26 N 2 OS, the structural formula is:
[0047]
[0048] The preparation method comprises the following steps:
[0049]
[0050] 1. Preparation of precursor dibenzothiophene-4-pinacol borate
[0051] The method was as described in step 1 of Example 1, and the reactant was replaced by dibenzothiophene from dibenzofuran to obtain white powder B, namely dibenzothiophene-4-pinacol borate.
[0052] 2. Preparation of precursor 2-(4-bromophenyl)-1-(4-methoxyphenyl)-phenanthroimidazole
[0053] Method is the same as step 2 of embodiment 1.
[0054] 3. Preparation of 2-(4-dibenzothiophene-4-phenyl)-1-(4-methoxyphenyl)phenanthroimidazole
[0055] The method is as described in step 3 of Example 1, except that the reactant is changed from product A to product B to obtain white powder E, namely 2-...
Embodiment 3
[0057] Preparation of non-doped electroluminescent device A by using dark blue organic hair material 2-(4-dibenzofuran-4-phenyl)-1-(4-methoxyphenyl)phenanthroimidazole as light-emitting layer material ,Specific steps are as follows:
[0058] 1. Clean the ITO glass in an organic solvent, dry it with nitrogen, and expose it to an ultraviolet-ozone atmosphere for 30 minutes.
[0059] 2. At 4.0 x 10 –4 Under the vacuum degree of Pa, the materials of the hole blocking layer, the hole transport layer, the light emitting layer and the electron transport layer are sequentially evaporated at a rate of 0.1-0.2nm / s, and finally LiF / Al is used as the cathode. The structure of the device is as follows: ITO / MoOx(2nm) / NPB(40nm) / luminescent material D(30nm) / TPBi(40nm) / LiF(1nm) / Al(100nm), see Image 6 Middle (a). Among them, NPB is N,N'-diphenyl-N,N'-(1-naphthyl)-1,1'-biphenyl-4,4'-diamine; TPBi is 1,3,5-tris (1-Phenyl-1H-benzimidazol-2-yl)benzene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com